Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial
- PMID: 30586757
- PMCID: PMC6416009
- DOI: 10.1161/CIRCULATIONAHA.118.037778
Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial
Abstract
Background: In the EMPA-REG OUTCOME trial (BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) in patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease, in comparison with placebo, empagliflozin reduced the risks of 3-point major adverse cardiovascular events (3-point MACE), cardiovascular and all-cause death, and hospitalization for heart failure. We investigated whether these effects varied across the spectrum of baseline cardiovascular risk.
Methods: Cardiovascular death, all-cause mortality, 3-point MACE, and hospitalization for heart failure in the pooled empagliflozin and placebo groups were analyzed in subgroups by prior myocardial infarction and stroke at baseline, and by estimated baseline cardiovascular risk based on the 10-point TIMI (Thrombolysis In Myocardial Infarction) Risk Score for Secondary Prevention.
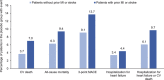
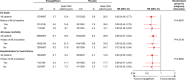
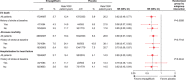
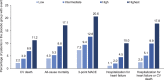
Results: Of 7020 patients who received the study drug, 65% had a prior myocardial infarction or stroke, and 12%, 40%, 30%, and 18% were at low, intermediate, high, and highest estimated cardiovascular risk according to TIMI Risk Score for Secondary Prevention (≤2, 3, 4, and ≥5 points, respectively). In the placebo group, 3-point MACE occurred during the trial in 7.3%, 9.4%, 12.6%, and 20.6% of patients at low, intermediate, high, and highest estimated baseline risk, respectively. Relative reductions in risk of cardiovascular death, all-cause mortality, 3-point MACE and hospitalization for heart failure with empagliflozin versus placebo were consistent in patients with and without prior myocardial infarction and/or stroke and across subgroups by TIMI Risk Score for Secondary Prevention at baseline ( P>0.05 for randomized group-by-subgroup interactions).
Conclusions: Despite all patients having atherosclerotic cardiovascular disease, patients in EMPA-REG OUTCOME demonstrated a broad risk spectrum for cardiovascular events. Reductions in key cardiovascular outcomes and mortality with empagliflozin versus placebo were consistent across the range of cardiovascular risk.
Clinical trial registration: URL: https://www.clinicaltrials.gov . Unique identifier: NCT01131676.
Keywords: cardiovascular diseases; carotid artery diseases; death, sudden, cardiac; diabetes mellitus, type 2; sodium-glucose transporter 2.
Figures


References
-
- Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, Popma JJ, Stevenson W. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006;114:2850–2870. doi: 10.1161/CIRCULATIONAHA.106.655688. - PubMed
-
- Alberts MJ, Bhatt DL, Mas JL, Ohman EM, Hirsch AT, Röther J, Salette G, Goto S, Smith SC, Jr, Liau CS, Wilson PW, Steg PG REduction of Atherothrombosis for Continued Health Registry Investigators. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J. 2009;30:2318–2326. doi: 10.1093/eurheartj/ehp355. - PMC - PubMed
-
- Khan H, Kalogeropoulos AP, Zannad F, Marti CN, Wilson PW, Georgiopoulou VV, Kanaya AM, Newman AB, Schelbert E, Harris TB, Kritchevsky S, Yancy C, Gheorghiade M, Fonarow GC, Butler J Health ABC Study. Incident heart failure in relation to vascular disease: insights from the Health, Aging, and Body Composition Study. Eur J Heart Fail. 2014;16:526–534. doi: 10.1002/ejhf.69. - PMC - PubMed
-
- Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Med. 2017;130(6S):S40–S50. doi: 10.1016/j.amjmed.2017.04.010. - PubMed
-
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

