Treatment effectiveness of a mindfulness-based inpatient group psychotherapy in adolescent substance use disorder - study protocol for a randomized controlled trial
- PMID: 30587217
- PMCID: PMC6307182
- DOI: 10.1186/s13063-018-3048-y
Treatment effectiveness of a mindfulness-based inpatient group psychotherapy in adolescent substance use disorder - study protocol for a randomized controlled trial
Abstract
Background: Current treatments for adolescents with substance use disorder (SUD) have had only limited success. In recent years, research has underlined the role of self-regulatory processes and impulsivity in the development and maintenance of SUD in adolescents. Mindfulness has gained much attention due to its capacity to influence self-regulatory processes, particularly in adult populations. Initial studies have shown the potential of mindfulness-based approaches in younger SUD patients. The aim of the present clinical trial is to evaluate the added treatment effect of a mindfulness-based group psychotherapy ("Mind it!") for adolescents with SUD in comparison to the current standard treatment. Moreover, we seek to explore the feasibility of the intervention and possible mediators of treatment effects.
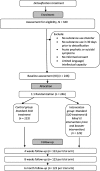
Methods/design: There will be N = 340 participants aged between 13 and 19 years who are receiving child or adolescent psychiatric or psychotherapeutic inpatient or day treatment targeting their SUD and who have reported substance use 30 days before detoxification and do not show acute psychotic or suicidal symptoms at baseline. The study is a prospective randomized controlled multi-center trial in which patients are assessed: (1) after completing a prior detoxification phase (t0), (2) at 4 weeks (t1), (3) at 8 weeks (t2), and (4) at 6 months after t2 (t3). Participants in the intervention group will receive mindfulness-based group psychotherapy in addition to their existing treatment regime. The primary outcome is substance use in the past 30 days at follow-up based on the Timeline Followback self-report. Secondary outcomes include craving, severity of dependence, and abstinence motivation. Mindfulness, impulsivity, and emotion regulation will be analyzed as possible mediators of treatment effects.
Discussion: This trial is expected to provide evidence of the added effect of a novel, safe, and feasible treatment option for adolescents with SUD.
Trial registration: German Register of Clinical Studies, DRKS00014041 . Registered on 17 April 2018.
Keywords: Adolescents; Child and adolescent psychiatry and psychotherapy; Group psychotherapy; Mindfulness-based intervention; Substance use disorder.
Conflict of interest statement
Ethics approval
The procedures of the current study have been approved by the ethics committee of Ruhr University Bochum (17–6268).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, McGrath J, Flaxman AD, Engell RE, Freedman GD, Whiteford HA. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One. 2013;8:e76635. doi: 10.1371/journal.pone.0076635. - DOI - PMC - PubMed
-
- Wittchen HU, Behrendt S, Hofler M, Perkonigg A, Lieb R, Buhringer G, Beesdo K. What are the high risk periods for incident substance use and transitions to abuse and dependence? Implications for early intervention and prevention. Int J Methods Psychiatr Res. 2008;17(Suppl 1):S16–S29. doi: 10.1002/mpr.254. - DOI - PMC - PubMed
-
- EMCDDA, editor. Statistical Bulletin: Treatment Demand Indicator. Luxembourg: Publications Office of the European Union; 2011.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

