Risk of Plasmodium vivax parasitaemia after Plasmodium falciparum infection: a systematic review and meta-analysis
- PMID: 30587297
- PMCID: PMC6300482
- DOI: 10.1016/S1473-3099(18)30596-6
Risk of Plasmodium vivax parasitaemia after Plasmodium falciparum infection: a systematic review and meta-analysis
Abstract
Background: A 14-day course of primaquine is used for radical cure of Plasmodium vivax and Plasmodium ovale malaria only. We quantified the risk of P vivax parasitaemia after treatment of Plasmodium falciparum with commonly used antimalarial drugs to assess the potential benefits of radical cure for all patients with uncomplicated malaria in co-endemic regions.
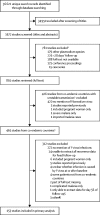
Methods: In this systematic review and meta-analysis, we searched MEDLINE, Embase, Web of Science, and the Cochrane Database of Systematic Reviews for prospective clinical studies in any language, published between Jan 1, 1960, and Jan 5, 2018, assessing drug efficacy in patients with uncomplicated P falciparum malaria in countries co-endemic for P vivax. Studies were included if the presence or absence of P vivax parasitaemia was recorded after treatment. The primary outcome was the risk of P vivax parasitaemia between day 7 and day 42 after initiation of antimalarial treatment for P falciparum, with the pooled risk calculated by random-effects meta-analysis. We compared the risk of P vivax parasitaemia after treatment with different artemisinin-based combination therapies (ACTs). This study is registered with PROSPERO, number CRD42017064838.
Findings: 153 of 891 screened studies were included in the analysis, including 31 262 patients from 323 site-specific treatment groups: 130 (85%) studies were from the Asia-Pacific region, 16 (10%) from the Americas, and seven (5%) from Africa. The risk of P vivax parasitaemia by day 42 was 5·6% (95% CI 4·0-7·4; I2=92·0%; 117 estimates). The risk of P vivax parasitaemia was 6·5% (95% CI 4·6-8·6) in regions of short relapse periodicity compared with 1·9% (0·4-4·0) in regions of long periodicity, and was greater after treatment with a more rapidly eliminated ACT: 15·3% (5·1-29·3) for artemether-lumefantrine compared with 4·5% (1·2-9·3) for dihydroartemisinin-piperaquine and 5·2% (2·9-7·9) for artesunate-mefloquine. Recurrent parasitaemia was delayed in patients treated with ACTs containing mefloquine or piperaquine compared with artemether-lumefantrine, but by day 63 the risk of vivax parasitaemia was more than 15% for all ACTs assessed.
Interpretation: Our findings show a high risk of vivax parasitaemia after treatment of falciparum malaria, particularly in areas with short relapse periodicity and after rapidly eliminated treatment. In co-endemic regions, universal radical cure for all patients with uncomplicated malaria has the potential to substantially reduce recurrent malaria.
Funding: Australian National Health and Medical Research Council, Royal Australasian College of Physicians, Wellcome Trust, and Bill & Melinda Gates Foundation.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures




Comment in
-
Primaquine for all: is it time to simplify malaria treatment in co-endemic areas?Lancet Infect Dis. 2019 Jan;19(1):10-12. doi: 10.1016/S1473-3099(18)30612-1. Lancet Infect Dis. 2019. PMID: 30587279 No abstract available.
References
-
- WHO . World Health Organization; Geneva: 2017. World malaria report 2017.https://www.who.int/malaria/publications/world-malaria-report-2017/en/
-
- Looareesuwan S, White NJ, Chittamas S, Bunnag D, Harinasuta T. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet. 1987;2:1052–1055. - PubMed
-
- Douglas NM, John GK, von Seidlein L, Anstey NM, Price RN. Chemotherapeutic strategies for reducing transmission of Plasmodium vivax malaria. Adv Parasitol. 2012;80:271–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

