A randomized phase II study of cabozantinib versus weekly paclitaxel in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study
- PMID: 30587441
- PMCID: PMC6542283
- DOI: 10.1016/j.ygyno.2018.12.008
A randomized phase II study of cabozantinib versus weekly paclitaxel in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study
Abstract
Introduction: Cabozantinib is a receptor tyrosine kinases inhibitor that targets MET (c-MET), VEGF receptor 2 (VEGFR2), RET, AXL, KIT, FLT-3, and TIE-2 and previously showed promising single agent activity in recurrent ovarian cancer.
Methods: This was an open label, 1:1 randomized study of cabozantinib 60 mg orally (PO) daily versus weekly paclitaxel 80 mg/m2 given 3 out of 4 weeks (NCT01716715); 111 patients were enrolled. Eligibility included persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma and at least one but no >3 prior chemotherapy regimens.
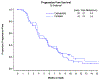
Results: Median PFS was similar for both treatment groups and was 5.3 months for cabozantinib and 5.5 months for weekly paclitaxel (HR 1.11 (90% CI 0.77-1.61, p = 0.64)). Secondary analyses of overall survival (OS) and event free survival (EFS) showed that cabozantinib did not perform as well as weekly paclitaxel. Median OS for cabozantinib was 19.4 months and was not reached for weekly paclitaxel (HR 2.27 (90% CI 1.17-4.41, p = 0.04). EFS was also worse in the cabozantinib arm, 3.5 months, compared to weekly paclitaxel at 5.0 months (HR 1.81 (90% CI 1.24-2.63, p = 0.01). Overall response rate (ORR) was less for cabozantinib compared to weekly paclitaxel (7% versus 24.1%). Gastrointestinal toxicities, specifically nausea, diarrhea, and abdominal pain were worse in the cabozantinib arm.
Conclusions: Median PFS was similar for cabozantinib and weekly paclitaxel. However, OS, EFS, and ORR were worse for cabozantinib compared to weekly paclitaxel. Cabozantinib given at this dose and schedule cannot be recommended as a treatment for recurrent ovarian cancer.
Keywords: Anti-vascular; Cabozantinib; Ovarian cancer; Paclitaxel.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICTS OF INTEREST
Dr. Ursula Matulonis received monies from Clearity Foundation and Ovarian Cancer Research Foundation for Board membership. She also served as a consultant and received monies from Merck, Fujifilm, Mersana, Geneos and Immunogen. She also served as a consultant for 2X Oncology, receiving no monies paid to herself. She has grants/grants pending from Merck for an investigator-initiated clinical trial. She received payment for development of education presentations from i3 Health.
Dr. Vicki Makker received monies paid to her for consultancy from Eisai Pharmaceuticals, Merck Pharmaceuticals and Karyopharm.
Dr. David Mutch received monies paid to him for consultancy from Tesaro.
Dr. Robert Mannel served on the Advisory Board as a consultancy for Clovis and Tesaro, receiving monies paid to his institution.
Dr. Carol Aghajanian served as Honorarium, Ad Boards for Tesaro, Mateon Therapeutics, Cerulean and ImmunoGen. She also served as Honorarium for Steering Committee Meeting for Mateon Therapeutics and Clovis.
All other co-authors have no conflicts of interest to declare.
Figures


References
-
- Siegal RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018; 68:7–30. - PubMed
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996; 334(1):1–6. - PubMed
-
- Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012. June 10; 30(17):2039–45. - PMC - PubMed
-
- Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017; 18:779–791. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

