Autophagic degradation of lamins facilitates the nuclear egress of herpes simplex virus type 1
- PMID: 30587512
- PMCID: PMC6363456
- DOI: 10.1083/jcb.201801151
Autophagic degradation of lamins facilitates the nuclear egress of herpes simplex virus type 1
Abstract
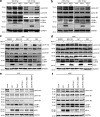
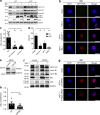
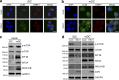
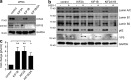
Dendritic cells (DCs) are crucial for the induction of potent antiviral immune responses. In contrast to immature DCs (iDCs), mature DCs (mDCs) are not permissive for infection with herpes simplex virus type 1 (HSV-1). Here, we demonstrate that HSV-1 infection of iDCs and mDCs induces autophagy, which promotes the degradation of lamin A/C, B1, and B2 in iDCs only. This in turn facilitates the nuclear egress of progeny viral capsids and thus the formation of new infectious particles. In contrast, lamin protein levels remain stable in HSV-1-infected mDCs due to an inefficient autophagic flux. Elevated protein levels of KIF1B and KIF2A in mDCs inhibited lamin degradation, likely by hampering autophagosome-lysosome fusion. Therefore, in mDCs, fewer progeny capsids were released from the nuclei into the cytosol, and fewer infectious virions were assembled. We hypothesize that inhibition of autophagic lamin degradation in mDCs represents a very powerful cellular counterstrike to inhibit the production of progeny virus and thus viral spread.
© 2019 Turan et al.
Figures



Comment in
-
Dendritic cells mature to resist lamin degradation and herpes virus release.J Cell Biol. 2019 Feb 4;218(2):387-388. doi: 10.1083/jcb.201812051. Epub 2019 Jan 15. J Cell Biol. 2019. PMID: 30647098 Free PMC article.
References
-
- Bodemann B.O., Orvedahl A., Cheng T., Ram R.R., Ou Y.H., Formstecher E., Maiti M., Hazelett C.C., Wauson E.M., Balakireva M., et al. . 2011. RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell. 144:253–267. 10.1016/j.cell.2010.12.018 - DOI - PMC - PubMed
-
- Budida R., Stankov M.V., Döhner K., Buch A., Panayotova-Dimitrova D., Tappe K.A., Pohlmann A., Sodeik B., and Behrens G.M.N.. 2017. Herpes simplex virus 1 interferes with autophagy of murine dendritic cells and impairs their ability to stimulate CD8+ T lymphocytes. Eur. J. Immunol. 47:1819–1834. 10.1002/eji.201646908 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

