Mupirocin for Staphylococcus aureus Decolonization of Infants in Neonatal Intensive Care Units
- PMID: 30587533
- PMCID: PMC6317770
- DOI: 10.1542/peds.2018-1565
Mupirocin for Staphylococcus aureus Decolonization of Infants in Neonatal Intensive Care Units
Abstract
: media-1vid110.1542/5849573989001PEDS-VA_2018-1565Video Abstract BACKGROUND AND OBJECTIVES: Staphylococcus aureus (SA) is the second leading cause of late-onset sepsis among infants in the NICU. Because colonization of nasal mucosa and/or skin frequently precedes invasive infection, decolonization strategies, such as mupirocin application, have been attempted to prevent clinical infection, but data supporting this approach in infants are limited. We conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants.
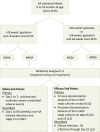
Methods: Between April 2014 and May 2016, infants <24 months old in the NICU at 8 study centers underwent serial screening for nasal SA. Colonized infants who met eligibility criteria were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Mupirocin effects on primary (day 8) and persistent (day 22) decolonization at all three body sites were assessed.
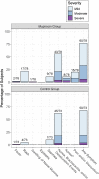
Results: A total of 155 infants were randomly assigned. Mupirocin was generally well tolerated, but rashes (usually mild and perianal) occurred significantly more often in treated versus untreated infants. Primary decolonization occurred in 62 of 66 (93.9%) treated infants and 3 of 64 (4.7%) control infants (P < .001). Twenty-one of 46 (45.7%) treated infants were persistently decolonized compared with 1 of 48 (2.1%) controls (P < .001).
Conclusions: Application of mupirocin to multiple body sites was safe and efficacious in eradicating SA carriage among infants in the NICU; however, after 2 to 3 weeks, many infants who remained hospitalized became recolonized.
Trial registration: ClinicalTrials.gov NCT01827358.
Copyright © 2019 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Kotloff reports funds to conduct research on rotavirus vaccine in Africa from Merck, Sharpe, and Dohme; Dr Creech reports grant funding and personal consultation fees from GlaxoSmithKline and Pfizer for staphylococcal vaccine development; Dr Harrison reports grants from Merck and GlaxoSmithKline and consultation fees from Pfizer; Dr Pahud reports grant funding from Pfizer and consultation fees from Pfizer, Sanofi, and Sequiris; Dr Bernstein reports consultation fees from Merck and Takeda; Dr Anderson reports funds to conduct clinical research from MedImmune, Regeneron, Pfizer, and Novavax and personal fees from AbbVie; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures

References
-
- Vergnano S, Menson E, Smith Z, et al. . Characteristics of invasive Staphylococcus aureus in United Kingdom neonatal units [published correction appears in Pediatr Infect Dis J. 2013;32(5):e226]. Pediatr Infect Dis J. 2011;30(10):850–854 - PubMed
-
- Carey AJ, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000-2007. J Perinatol. 2010;30(2):135–139 - PubMed
-
- Shane AL, Hansen NI, Stoll BJ, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129(4). Available at: www.pediatrics.org/cgi/content/full/129/4/e914 - PMC - PubMed
-
- Stoll BJ, Hansen NI, Adams-Chapman I, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

