ATP binding without hydrolysis switches sulfonylurea receptor 1 (SUR1) to outward-facing conformations that activate KATP channels
- PMID: 30587573
- PMCID: PMC6416425
- DOI: 10.1074/jbc.RA118.005236
ATP binding without hydrolysis switches sulfonylurea receptor 1 (SUR1) to outward-facing conformations that activate KATP channels
Abstract
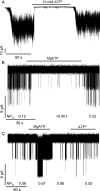
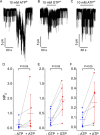
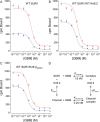
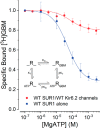
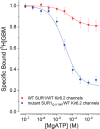
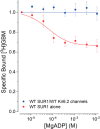
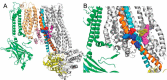
Neuroendocrine-type ATP-sensitive K+ (KATP) channels are metabolite sensors coupling membrane potential with metabolism, thereby linking insulin secretion to plasma glucose levels. They are octameric complexes, (SUR1/Kir6.2)4, comprising sulfonylurea receptor 1 (SUR1 or ABCC8) and a K+-selective inward rectifier (Kir6.2 or KCNJ11). Interactions between nucleotide-, agonist-, and antagonist-binding sites affect channel activity allosterically. Although it is hypothesized that opening these channels requires SUR1-mediated MgATP hydrolysis, we show here that ATP binding to SUR1, without hydrolysis, opens channels when nucleotide antagonism on Kir6.2 is minimized and SUR1 mutants with increased ATP affinities are used. We found that ATP binding is sufficient to switch SUR1 alone between inward- or outward-facing conformations with low or high dissociation constant, KD , values for the conformation-sensitive channel antagonist [3H]glibenclamide ([3H]GBM), indicating that ATP can act as a pure agonist. Assembly with Kir6.2 reduced SUR1's KD for [3H]GBM. This reduction required the Kir N terminus (KNtp), consistent with KNtp occupying a "transport cavity," thus positioning it to link ATP-induced SUR1 conformational changes to channel gating. Moreover, ATP/GBM site coupling was constrained in WT SUR1/WT Kir6.2 channels; ATP-bound channels had a lower KD for [3H]GBM than ATP-bound SUR1. This constraint was largely eliminated by the Q1179R neonatal diabetes-associated mutation in helix 15, suggesting that a "swapped" helix pair, 15 and 16, is part of a structural pathway connecting the ATP/GBM sites. Our results suggest that ATP binding to SUR1 biases KATP channels toward open states, consistent with SUR1 variants with lower KD values causing neonatal diabetes, whereas increased KD values cause congenital hyperinsulinism.
Keywords: ABC transporter; ATP-binding cassette transporter subfamily C member 8 (ABCC8); ATP-sensitive potassium channel; KATP channel; KCNJ11 (Kir6.2); SUR1; allosteric regulation; congenital hyperinsulinism; diabetes; glibenclamide; hyperinsulinism; ion channel; metabolic sensor; neonatal diabetes.
© 2019 Sikimic et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Two neonatal diabetes mutations on transmembrane helix 15 of SUR1 increase affinity for ATP and ADP at nucleotide binding domain 2.J Biol Chem. 2012 May 25;287(22):17985-95. doi: 10.1074/jbc.M112.349019. Epub 2012 Mar 27. J Biol Chem. 2012. PMID: 22451668 Free PMC article.
-
Sulfonylureas suppress the stimulatory action of Mg-nucleotides on Kir6.2/SUR1 but not Kir6.2/SUR2A KATP channels: a mechanistic study.J Gen Physiol. 2014 Nov;144(5):469-86. doi: 10.1085/jgp.201411222. J Gen Physiol. 2014. PMID: 25348414 Free PMC article.
-
The Kir6.2-F333I mutation differentially modulates KATP channels composed of SUR1 or SUR2 subunits.J Physiol. 2007 Jun 15;581(Pt 3):1259-69. doi: 10.1113/jphysiol.2007.130211. Epub 2007 Mar 29. J Physiol. 2007. PMID: 17395632 Free PMC article.
-
Molecular biology of adenosine triphosphate-sensitive potassium channels.Endocr Rev. 1999 Apr;20(2):101-35. doi: 10.1210/edrv.20.2.0361. Endocr Rev. 1999. PMID: 10204114 Review.
-
Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator.Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):257-67. doi: 10.1098/rstb.2008.0142. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18990670 Free PMC article. Review.
Cited by
-
Mechanism of pharmacochaperoning in a mammalian KATP channel revealed by cryo-EM.Elife. 2019 Jul 25;8:e46417. doi: 10.7554/eLife.46417. Elife. 2019. PMID: 31343405 Free PMC article.
-
Clinical and Genetic Characteristics of ABCC8 Nonneonatal Diabetes Mellitus: A Systematic Review.J Diabetes Res. 2021 Sep 30;2021:9479268. doi: 10.1155/2021/9479268. eCollection 2021. J Diabetes Res. 2021. PMID: 34631896 Free PMC article.
-
ATP modulation of osmotically activated anionic current in the membrane of Phycomyces blakesleeanus sporangiophore.Sci Rep. 2023 Jul 24;13(1):11897. doi: 10.1038/s41598-023-39021-9. Sci Rep. 2023. PMID: 37488205 Free PMC article.
-
Dynamic duo: Kir6 and SUR in KATP channel structure and function.Channels (Austin). 2024 Dec;18(1):2327708. doi: 10.1080/19336950.2024.2327708. Epub 2024 Mar 15. Channels (Austin). 2024. PMID: 38489043 Free PMC article. Review.
-
Possible New Strategies for the Treatment of Congenital Hyperinsulinism.Front Endocrinol (Lausanne). 2020 Oct 27;11:545638. doi: 10.3389/fendo.2020.545638. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33193079 Free PMC article.
References
-
- Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P. 4th, Boyd A. E. 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., and Nelson D. A. (1995) Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268, 423–426 10.1126/science.7716547 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

