O-GlcNAcylation regulates integrin-mediated cell adhesion and migration via formation of focal adhesion complexes
- PMID: 30587575
- PMCID: PMC6398131
- DOI: 10.1074/jbc.RA118.005923
O-GlcNAcylation regulates integrin-mediated cell adhesion and migration via formation of focal adhesion complexes
Abstract
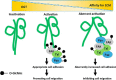
O-GlcNAcylation is a post-translational modification of a protein serine or threonine residue catalyzed by O-GlcNAc transferase (OGT) in the nucleus and cytoplasm. O-GlcNAcylation plays important roles in the cellular signaling that affect the different biological functions of cells, depending upon cell type. However, whether or not O-GlcNAcylation regulates cell adhesion and migration remains unclear. Here, we used the doxycycline-inducible short hairpin RNA (shRNA) system to establish an OGT knockdown (KD) HeLa cell line and found that O-GlcNAcylation is a key regulator for cell adhesion, migration, and focal adhesion (FA) complex formation. The expression levels of OGT and O-GlcNAcylation were remarkably suppressed 24 h after induction of doxycycline. Knockdown of OGT significantly promoted cell adhesion, but it suppressed the cell migration on fibronectin. The immunostaining with paxillin, a marker for FA plaque, clearly showed that the number of FAs was increased in the KD cells compared with that in the control cells. The O-GlcNAcylation levels of paxillin, talin, and focal adhesion kinase were down-regulated in KD cells. Interestingly, the complex formation between integrin β1, focal adhesion kinase, paxillin, and talin was greatly increased in KD cells. Consistently, levels of active integrin β1 were significantly enhanced in KD cells, whereas they were decreased in cells overexpressing OGT. The data suggest a novel regulatory mechanism for O-GlcNAcylation during FA complex formation, which thereby affects integrin activation and integrin-mediated functions such as cell adhesion and migration.
Keywords: O-GlcNAcylation; O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT); cell adhesion; cell migration; focal adhesions; glycosylation; integrin.
© 2019 Xu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
O-GlcNAcylation regulates β1,4-GlcNAc-branched N-glycan biosynthesis via the OGT/SLC35A3/GnT-IV axis.FASEB J. 2022 Feb;36(2):e22149. doi: 10.1096/fj.202101520R. FASEB J. 2022. PMID: 34981577
-
O-GlcNAcylation of Focal Adhesion Kinase Regulates Cell Adhesion, Migration, and Proliferation via the FAK/AKT Pathway.Biomolecules. 2024 Dec 10;14(12):1577. doi: 10.3390/biom14121577. Biomolecules. 2024. PMID: 39766284 Free PMC article.
-
Elevated O-GlcNAcylation promotes gastric cancer cells proliferation by modulating cell cycle related proteins and ERK 1/2 signaling.Oncotarget. 2016 Sep 20;7(38):61390-61402. doi: 10.18632/oncotarget.11359. Oncotarget. 2016. PMID: 27542217 Free PMC article.
-
O-GlcNAcylation in cancer development and immunotherapy.Cancer Lett. 2023 Jul 10;566:216258. doi: 10.1016/j.canlet.2023.216258. Epub 2023 Jun 4. Cancer Lett. 2023. PMID: 37279852 Review.
-
OGT: a short overview of an enzyme standing out from usual glycosyltransferases.Biochem Soc Trans. 2017 Apr 15;45(2):365-370. doi: 10.1042/BST20160404. Biochem Soc Trans. 2017. PMID: 28408476 Review.
Cited by
-
Crosstalk between O-GlcNAcylation and phosphorylation in metabolism: regulation and mechanism.Cell Death Differ. 2025 Jul;32(7):1181-1199. doi: 10.1038/s41418-025-01473-z. Epub 2025 Mar 5. Cell Death Differ. 2025. PMID: 40045035 Review.
-
β1 integrin is a sensor of blood flow direction.J Cell Sci. 2019 Jun 3;132(11):jcs229542. doi: 10.1242/jcs.229542. J Cell Sci. 2019. PMID: 31076511 Free PMC article.
-
Integrin β1 in Pancreatic Cancer: Expressions, Functions, and Clinical Implications.Cancers (Basel). 2022 Jul 11;14(14):3377. doi: 10.3390/cancers14143377. Cancers (Basel). 2022. PMID: 35884437 Free PMC article. Review.
-
Role of Site-Specific Glycosylation in the I-Like Domain of Integrin β1 in Small Extracellular Vesicle-Mediated Malignant Behavior and FAK Activation.Int J Mol Sci. 2021 Feb 10;22(4):1770. doi: 10.3390/ijms22041770. Int J Mol Sci. 2021. PMID: 33578954 Free PMC article.
-
O-GlcNAcylation homeostasis controlled by calcium influx channels regulates multiple myeloma dissemination.J Exp Clin Cancer Res. 2021 Mar 16;40(1):100. doi: 10.1186/s13046-021-01876-z. J Exp Clin Cancer Res. 2021. PMID: 33726758 Free PMC article.
References
-
- Itkonen H. M., Minner S., Guldvik I. J., Sandmann M. J., Tsourlakis M. C., Berge V., Svindland A., Schlomm T., and Mills I. G. (2013) O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 73, 5277–5287 10.1158/0008-5472.CAN-13-0549 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

