Toward understanding cancer stem cell heterogeneity in the tumor microenvironment
- PMID: 30587589
- PMCID: PMC6320545
- DOI: 10.1073/pnas.1815345116
Toward understanding cancer stem cell heterogeneity in the tumor microenvironment
Abstract
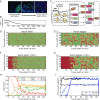
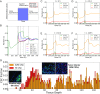
The epithelial-mesenchymal transition (EMT) and cancer stem cell (CSC) formation are two paramount processes driving tumor progression, therapy resistance, and cancer metastasis. Recent experiments show that cells with varying EMT and CSC phenotypes are spatially segregated in the primary tumor. The underlying mechanisms generating such spatiotemporal dynamics in the tumor microenvironment, however, remain largely unexplored. Here, we show through a mechanism-based dynamical model that the diffusion of EMT-inducing signals such as TGF-β, together with noncell autonomous control of EMT and CSC decision making via the Notch signaling pathway, can explain experimentally observed disparate localization of subsets of CSCs with varying EMT phenotypes in the tumor. Our simulations show that the more mesenchymal CSCs lie at the invasive edge, while the hybrid epithelial/mesenchymal (E/M) CSCs reside in the tumor interior. Further, motivated by the role of Notch-Jagged signaling in mediating EMT and stemness, we investigated the microenvironmental factors that promote Notch-Jagged signaling. We show that many inflammatory cytokines such as IL-6 that can promote Notch-Jagged signaling can (i) stabilize a hybrid E/M phenotype, (ii) increase the likelihood of spatial proximity of hybrid E/M cells, and (iii) expand the fraction of CSCs. To validate the predicted connection between Notch-Jagged signaling and stemness, we knocked down JAG1 in hybrid E/M SUM149 human breast cancer cells in vitro. JAG1 knockdown significantly restricted tumor organoid formation, confirming the key role that Notch-Jagged signaling can play in tumor progression. Together, our integrated computational-experimental framework reveals the underlying principles of spatiotemporal dynamics of EMT and CSCs.
Keywords: Notch signaling; breast tumor organoids; cancer stem cells; epithelial–mesenchymal transition; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

