Pre-pubertal exposure to high temperature impairs ovarian and adrenal gland function in female rats
- PMID: 30587674
- PMCID: PMC6395223
- DOI: 10.1292/jvms.18-0644
Pre-pubertal exposure to high temperature impairs ovarian and adrenal gland function in female rats
Abstract
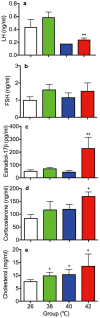
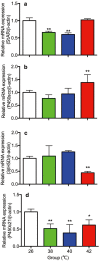
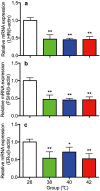
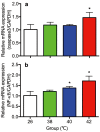
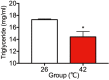
The influence of different levels of heat exposure on the functions of ovarian and adrenal gland were investigated in pre-puberty female rats. Three-week old female rats were treated with control (26°C) or three higher temperatures (38, 40 and 42°C) for 2hr/day. After 9 days of treatment, blood samples were collected for measurement of luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol-17β, corticosterone, cholesterol and triglyceride. Adrenal glands, ovaries and liver were collected for analyzing gene expressions. Body and liver weight were significantly low in the 42°C heating group. Circulating LH and triglyceride in the 42°C heating group were significantly lower, and estradiol-17β, corticosterone and cholesterol were significantly higher than those of the control group. The gene expression of 3β-HSD and P450c21 in the adrenal gland; 3β-HSD, receptors of LH, FSH and estrogen in the ovary were significantly low in heated rats. The liver gene expressions of caspase 3 and NK-κB were significantly high in 42°C heated rats, suggesting that the ability of liver metabolic function reduced in the 42°C heated rats. These results demonstrated that the high temperature is responsible for suppression of ovarian function by decreasing the expression of steroidogenic enzymes, estrogen and gonadotropin receptors in the ovary. Increase in circulating estradiol-17β in the heated rats may be due to accumulate this hormone in circulation by potential changes in liver metabolism during the heat stress.
Keywords: corticosterone; estradiol-17β; heat stress; rat; steroidogenesis.
Figures



Similar articles
-
Altered corticosterone status impairs steroidogenesis in the granulosa and thecal cells of Wistar rats.J Steroid Biochem Mol Biol. 2000 Jun;73(3-4):153-8. doi: 10.1016/s0960-0760(00)00063-7. J Steroid Biochem Mol Biol. 2000. PMID: 10925215
-
A quantitative analysis of the maturation of steroid negative feedbacks controlling gonadotropin release in the female rat: the infantile-juvenile periods, transition from an androgenic to a predominantly estrogenic control.Endocrinology. 1981 Apr;108(4):1313-20. doi: 10.1210/endo-108-4-1313. Endocrinology. 1981. PMID: 6781871
-
Luteinizing-hormone-mediated precocious puberty induced in female rats by a prepuberal pituitary graft.Neuroendocrinology. 1989 Nov;50(5):495-9. doi: 10.1159/000125270. Neuroendocrinology. 1989. PMID: 2514388
-
Interrelationships between the hypothalamus, pituitary gland, ovary, adrenal gland, and the open period for LH release in the hen (Gallus domesticus).J Exp Zool. 1984 Dec;232(3):501-11. doi: 10.1002/jez.1402320317. J Exp Zool. 1984. PMID: 6394694 Review.
-
Sexual maturation and the adrenal glands.J Reprod Fertil. 1978 Mar;52(2):413-8. doi: 10.1530/jrf.0.0520413. J Reprod Fertil. 1978. PMID: 344876 Review. No abstract available.
Cited by
-
Allostasis in Neuroendocrine Systems Controlling Reproduction.Endocrinology. 2023 Aug 28;164(10):bqad125. doi: 10.1210/endocr/bqad125. Endocrinology. 2023. PMID: 37586095 Free PMC article. Review.
-
Integrative Analysis of Vaginal Microorganisms and Serum Metabolomics in Rats With Estrous Cycle Disorder Induced by Long-Term Heat Exposure Based on 16S rDNA Gene Sequencing and LC/MS-Based Metabolomics.Front Cell Infect Microbiol. 2021 Mar 2;11:595716. doi: 10.3389/fcimb.2021.595716. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33738264 Free PMC article.
-
Pathophysiological Changes in Female Rats with Estrous Cycle Disorder Induced by Long-Term Heat Stress.Biomed Res Int. 2020 Jun 17;2020:4701563. doi: 10.1155/2020/4701563. eCollection 2020. Biomed Res Int. 2020. PMID: 32685488 Free PMC article.
-
Characteristics of menstrual cycle disorder and saliva metabolomics of young women in a high-temperature environment.Front Physiol. 2023 Jan 13;13:994990. doi: 10.3389/fphys.2022.994990. eCollection 2022. Front Physiol. 2023. PMID: 36714308 Free PMC article.
-
The impact of season, temperature, and direct normal irradiance on IVF pregnancy outcomes: a retrospective cohort study.Int J Biometeorol. 2025 Aug;69(8):2053-2065. doi: 10.1007/s00484-025-02951-2. Epub 2025 Jun 18. Int J Biometeorol. 2025. PMID: 40531342 Free PMC article.
References
-
- Black J. L., Mullan B. P., Lorschy M. L., Giles L. R.1993. Lactation in the sow during heat stress. Livest. Prod. Sci. 35: 153–170. doi: 10.1016/0301-6226(93)90188-N - DOI
-
- Bridges P. J., Brusie M. A., Fortune J. E.2005. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest. Anim. Endocrinol. 29: 508–522. doi: 10.1016/j.domaniend.2005.02.017 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

