Multiplex Lateral Flow Immunoassay: An Overview of Strategies towards High-throughput Point-of-Need Testing
- PMID: 30587769
- PMCID: PMC6468474
- DOI: 10.3390/bios9010002
Multiplex Lateral Flow Immunoassay: An Overview of Strategies towards High-throughput Point-of-Need Testing
Abstract
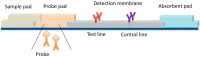
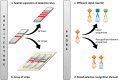
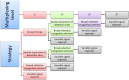
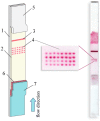
Simultaneous measurement of different substances from a single sample is an emerging issue for achieving efficient and high-throughput detection in several fields of application. Although immunoanalytical techniques have well-established and prevailing advantages over alternative screening analytical platforms, one of the incoming challenges for immunoassay is exact multiplexing. Lateral flow immunoassay (LFIA) is a leading immunoanalytical technique for onsite analysis, thanks to its simplicity, rapidity, and cost-effectiveness. Moreover, LFIA architecture is adaptable to multiplexing, and is therefore a possible answer to the pressing demand of multiplexing point-of-need analysis. This review presents an overview of diverse approaches for multiplex LFIA, with a special focus on strategies based on new types of magnetic, fluorescent, and colored labels.
Keywords: immunoassay; immunochromatographic test; point-of-care testing; rapid test.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
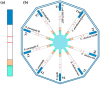
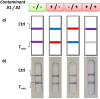
References
-
- Dhawan A. Point-of-Care Technologies: An Evolution in Personalized Healthcare. [(accessed on 15 September 2018)];IEEE Life Sciences eNewsletter. Available online: https://lifesciences.ieee.org/lifesciences-newsletter/2013/january-2013/...
-
- Sajid M., Kawde A.-N., Daud M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015;19:689–705. doi: 10.1016/j.jscs.2014.09.001. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

