Anti-Oxidant and Tyrosinase Inhibitory In Vitro Activity of Amino Acids and Small Peptides: New Hints for the Multifaceted Treatment of Neurologic and Metabolic Disfunctions
- PMID: 30587771
- PMCID: PMC6356958
- DOI: 10.3390/antiox8010007
Anti-Oxidant and Tyrosinase Inhibitory In Vitro Activity of Amino Acids and Small Peptides: New Hints for the Multifaceted Treatment of Neurologic and Metabolic Disfunctions
Abstract
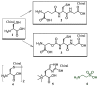
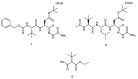
Oxidative damage is among the factors associated with the onset of chronic pathologies, such as neurodegenerative and metabolic diseases. Several classes of anti-oxidant compounds have been suggested as having a protective role against cellular stressors, but, in this perspective, peptides' world represents a poorly explored source. In the present study, the free radical scavenging properties, the metal ion reducing power, and the metal chelating activity of a series of sulfurated amino acids and tripeptides were determined in vitro through canonical assays (DPPH, ABTS, CUPRAC, FRAP, PM, and EECC) and estimated in comparison with the corresponding activities of synthetic peptide semicarbazones, incorporating the peculiar non-proteinogenic amino acid, tert-leucine (tLeu). The compounds exhibited remarkable anti-oxidant properties. As expected, sulfurated compounds 1⁻5 were found to be the most efficient radical scavengers and strongest reductants. Nevertheless, tLeu-containing peptides 7 and 8 disclosed notable metal reducing and chelating activities. These unprecedented results indicate that tLeu-featuring di- and tripeptide backbones, bearing the semicarbazone chelating moiety, are compatible with the emergence of an anti-oxidant potential. Additionally, when tested against a panel of enzymes usually targeted for therapeutic purposes in neurodegenerative and metabolic disorders, all samples were found to be good inhibitors of tyrosinase.
Keywords: anti-oxidant; metabolic disorders; neurodegenerative diseases; scavenger.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Carrasco-Pancorbo A., Cerretani L., Bendini A., Segura-Carretero A., Del Carlo M., Gallina-Toschi T., Lercker G., Compagnone D., Fernandez-Gutierrez A. Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J. Agric. Food Chem. 2005;53:8918–8925. doi: 10.1021/jf0515680. - DOI - PubMed
-
- Wang X., Wu Q., Liu A., Anadón A., Rodríguez J.-L., Martínez-Larrañaga M.-R., Yuan Z., Martínez M.-A. Paracetamol: Overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds in vivo and in vitro. Drug Metab. Rev. 2017;49:395–437. doi: 10.1080/03602532.2017.1354014. - DOI - PubMed
-
- Zengin G., Abdallah H.H., Dogan A., Mollica A., Aumeeruddy-Elalfi Z., Mahomoodally M.F. Phenolic components and assessment of biological properties of Tchihatchewia isatidea Boiss. extracts: Docking and functional approaches for designing novel products. Food Chem. Toxicol. 2018;111:423–431. doi: 10.1016/j.fct.2017.11.055. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous