Phase III trials examining the efficacy of cetirizine ophthalmic solution 0.24% compared to vehicle for the treatment of allergic conjunctivitis in the conjunctival allergen challenge model
- PMID: 30587908
- PMCID: PMC6296187
- DOI: 10.2147/OPTH.S185835
Phase III trials examining the efficacy of cetirizine ophthalmic solution 0.24% compared to vehicle for the treatment of allergic conjunctivitis in the conjunctival allergen challenge model
Abstract
Purpose: The purpose of these Phase III studies was to evaluate the efficacy and safety of cetirizine ophthalmic solution 0.24% compared with vehicle in the treatment of allergen-induced conjunctivitis using the Ora conjunctival allergen challenge (CAC)® model.
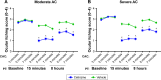
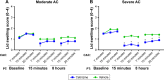
Methods: The single-center (Study 1) and multi-center (Study 2), double-masked, randomized, vehicle-controlled, parallel group, CAC studies were conducted over ~5 weeks and four study visits. The study design only differed in entry criteria: Study 2 required more severe allergic conjunctivitis symptoms. Subjects were screened for an allergen response at Visits 1 and 2 and then randomized at Visit 3. Approximately 100 subjects were randomized in each study. The primary efficacy endpoints were ocular itching and conjunctival redness 15 minutes and 8 hours post-treatment, post-CAC.
Results: Cetirizine treatment administered 15 minutes or 8 hours prior to CAC resulted in significantly lower ocular itching at all time points post-CAC (P<0.0001) compared to vehicle in both studies. Conjunctival redness measured by the investigator was significantly lower after cetirizine treatment compared to vehicle at 7 minutes post-CAC at both 15 minutes and 8 hours post-treatment in both studies (P<0.05). All secondary endpoints were in favor and confirmatory of cetirizine efficacy with significant improvement in chemosis, eyelid swelling, tearing, ciliary redness, and episcleral redness, as well as nasal symptoms (rhinorrhea, nasal pruritus, ear or palatal pruritus, and nasal congestion) post-CAC. The most robust treatment differences were observed in Study 2 where more severe symptoms were required for study entry (P<0.05). No safety concerns for cetirizine ophthalmic solution 0.24% were identified.
Conclusion: Cetirizine ophthalmic solution 0.24% was shown to be efficacious in the treatment of ocular and nasal signs and symptoms associated with allergic conjunctivitis and demonstrated a favorable safety profile. Clinical efficacy was demonstrated with a 15-minute onset of action and añ8-hour duration of action.
Keywords: cetirizine; ocular allergy; ocular itching; safety; topical administration.
Conflict of interest statement
Disclosure Paul Gomes is an employee of Ora, Inc. Mark C Jasek is an employee of Eyevance Pharmaceuticals. Zerviate is a proprietary trademark of Nicox SA, and is licensed by Eyevance Pharmaceuticals. Ora Conjunctival Allergen Challegen (Ora-CAC®) is a registered trademark of Ora, Inc. The other authors report no conflicts of interest in this work.
Figures

References
-
- Bielory L. Allergic and immunologic disorders of the eye. Part II: ocular allergy. J Allergy Clin Immunol. 2000;106(6):1019–1032. - PubMed
-
- Burbach GJ, Heinzerling LM, Röhnelt C, et al. Ragweed sensitization in Europe – GA(2)LEN study suggests increasing prevalence. Allergy. 2009;64(4):664–665. - PubMed
-
- Bacon AS, Ahluwalia P, Irani AM, et al. Tear and conjunctival changes during the allergen-induced early- and late-phase responses. J Allergy Clin Immunol. 2000;106(5):948–954. - PubMed
-
- Ciprandi G, Buscaglia S, Pesce GP, Bagnasco M, Canonica GW. Ocular challenge and hyperresponsiveness to histamine in patients with allergic conjunctivitis. J Allergy Clin Immunol. 1993;91(6):1227–1230. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

