Combination of rifaximin and lactulose improves clinical efficacy and mortality in patients with hepatic encephalopathy
- PMID: 30587923
- PMCID: PMC6301297
- DOI: 10.2147/DDDT.S172324
Combination of rifaximin and lactulose improves clinical efficacy and mortality in patients with hepatic encephalopathy
Abstract
Background: Rifaximin and lactulose are common effective agents for hepatic encephalopathy (HE). Whether a combination of rifaximin and lactulose improves the efficacy and mortality in patients with HE compared with lactulose alone needs to be analyzed.
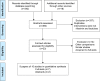
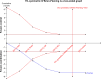
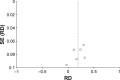
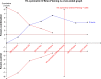
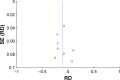
Methods: A systematic search was performed in electronic databases and other sources for possible studies focusing on combination therapy of rifaximin and lactulose for HE between January 2000 and February 2018. A meta-analysis was performed by the method recommended by the Cochrane Collaboration, and estimated effect size was presented as risk difference (RD), 95% CI, and the number needed to treat (NNT). Subgroup analysis, sensitivity analysis, and Trial Sequence Analysis were comprehensively performed to indicate the source of heterogeneity and risk of bias.
Results: Five randomized and five observational studies involving 2,276 patients were included. Combination therapy had a significant advantage in both clinical efficacy increase (RD 0.26, 95% CI 0.19-0.32, NNT 5) and mortality decrease (RD -0.16, 95% CI -0.20-0.11, NNT 9) in overall analysis. In the pooled analysis of randomized studies, combination therapy showed similar results in clinical efficacy (RD 0.25, 95% CI 0.16-0.35, NNT 4) and mortality (RD -0.22, 95% CI -0.33-0.12, NNT 5). Compared with lactulose, hospital stay was also reduced in combination therapy, and there was no significant difference in treatment-related adverse events between the two groups.
Conclusion: Combination of rifaximin and lactulose has beneficial effects on HE. Compared with lactulose alone, additional rifaximin increases clinical efficacy and decreases mortality. However, its effects on different types of HE are still uncertain.
Keywords: HE; combination therapy; lactulose; meta-analysis; rifaximin.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
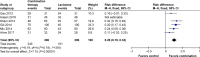
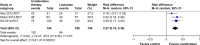

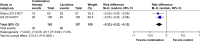

References
-
- Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735. - PubMed
-
- Riggio O, Ridola L, Pasquale C, et al. Evidence of persistent cognitive impairment after resolution of overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2011;9(2):181–183. - PubMed
-
- Pereira K, Carrion AF, Salsamendi J, Doshi M, Baker R, Kably I. Endovascular Management of Refractory Hepatic Encephalopathy Complication of Transjugular Intrahepatic Portosystemic Shunt (TIPS): Comprehensive Review and Clinical Practice Algorithm. Cardiovasc Intervent Radiol. 2016;39(2):170–182. - PubMed
-
- Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9):1034.e1–1041.e1. - PubMed
-
- Amodio P, Del Piccolo F, Pettenò E, et al. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol. 2001;35(1):37–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

