Efficacy and safety of a novel, nebulized glycopyrrolate for the treatment of COPD: effect of baseline disease severity and age; pooled analysis of GOLDEN 3 and GOLDEN 4
- PMID: 30587959
- PMCID: PMC6305132
- DOI: 10.2147/COPD.S184808
Efficacy and safety of a novel, nebulized glycopyrrolate for the treatment of COPD: effect of baseline disease severity and age; pooled analysis of GOLDEN 3 and GOLDEN 4
Abstract
Background: The efficacy and safety of nebulized glycopyrrolate inhalation solution (GLY), administered twice daily (BID) via the innovative eFlow® Closed System nebulizer (PARI Pharma GmbH, Starnberg, Germany), were demonstrated in two replicate, placebo-controlled, 12-week Phase III studies (GOLDEN 3 and GOLDEN 4). This report evaluates the efficacy and safety of GLY by baseline disease severity and age in the pooled GOLDEN 3 and GOLDEN 4 patient population (N=1,294).
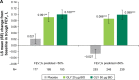
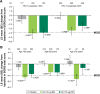
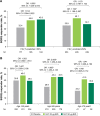
Methods: Patients were grouped by baseline predicted post-bronchodilator FEV1 (<50%, ≥50%) and age (<65, ≥65, ≥75 years).
Results: GLY (25 and 50 μg BID) produced significant improvements in trough FEV1 in FEV1% predicted <50% (0.070 L, 0.079 L) and ≥50% (0.112 L, 0.126 L) subgroups (P<0.01 vs placebo), and in patients aged <65 (0.056 L, 0.086 L), ≥65 (0.140 L, 0.124 L), and ≥75 (0.144 L, 0.120 L) years (P<0.05 vs placebo). St George's Respiratory Questionnaire (SGRQ) total score was significantly improved with GLY 25 and 50 μg BID (P<0.05 vs placebo) in FEV1% predicted <50% (-3.237, -3.061) and ≥50% (-3.392, -2.322) and in <65 years (-3.447, -2.318) and ≥65 years (-3.053, -3.098) subgroups. In patients aged ≥75 years, GLY 25 μg reduced SGRQ total score by -6.278 units (P<0.01 vs placebo). The incidence of treatment-emergent adverse events was similar between GLY and placebo across all subgroups, and the overall incidence of cardiovascular events was low.
Conclusions: Nebulized GLY improved lung function and health status and was well tolerated over 12 weeks in patients with moderate-to-very-severe COPD, irrespective of baseline disease severity and age.
Clinical trial registration: NCT02347761, NCT02347774.
Keywords: COPD; LAMA; age; disease severity; long-acting muscarinic antagonist; nebulized glycopyrrolate; nebulizer.
Conflict of interest statement
Disclosure Jill Ohar has served on advisory boards for Sunovion Pharmaceuticals Inc., AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Mylan, and Theravance, and has provided expert witness testimony for: Wallace & Graham, Levy Konigsberg, Goldenberg Heller & Antognoli, Simon Greensone Panatier Bartlett, Williams Kherkher Hart, Gori Julian & Associates, Simmons Hanley Conroy, and Elrod Pope. Robert Tosiello, Thomas Goodin, and Shahin Sanjar are employees of Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: Results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials.Respir Med. 2017 Nov;132:238-250. doi: 10.1016/j.rmed.2017.07.011. Epub 2017 Jul 19. Respir Med. 2017. PMID: 28838685 Clinical Trial.
-
Effect of background long-acting beta2-agonist therapy on the efficacy and safety of a novel, nebulized glycopyrrolate in subjects with moderate-to-very-severe COPD.Int J Chron Obstruct Pulmon Dis. 2018 Sep 19;13:2917-2929. doi: 10.2147/COPD.S172408. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30275690 Free PMC article. Clinical Trial.
-
Dose selection for glycopyrrolate/eFlow® phase III clinical studies: results from GOLDEN (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer) phase II dose-finding studies.Respir Res. 2017 Dec 4;18(1):202. doi: 10.1186/s12931-017-0681-z. Respir Res. 2017. PMID: 29202767 Free PMC article. Clinical Trial.
-
Glycopyrrolate/eFlow CS: The First Nebulized Long-Acting Muscarinic Antagonist Approved to Treat Chronic Obstructive Pulmonary Disease.Ann Pharmacother. 2019 Mar;53(3):285-293. doi: 10.1177/1060028018798753. Epub 2018 Sep 1. Ann Pharmacother. 2019. PMID: 30175596 Free PMC article. Review.
-
Role of combined indacaterol and glycopyrronium bromide (QVA149) for the treatment of COPD in Japan.Int J Chron Obstruct Pulmon Dis. 2015 Apr 21;10:813-22. doi: 10.2147/COPD.S56067. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 25960646 Free PMC article. Review.
Cited by
-
Improvement in Lung Function and Patient-Reported Outcomes in Patients with COPD with Comorbid Anxiety and Depression Receiving Nebulized Glycopyrrolate in the GOLDEN 3 and 4 Studies.Int J Chron Obstruct Pulmon Dis. 2021 Mar 31;16:865-875. doi: 10.2147/COPD.S294053. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 33833507 Free PMC article.
-
Impact of baseline clinical features on outcomes of nebulized glycopyrrolate therapy in COPD.NPJ Prim Care Respir Med. 2021 Oct 7;31(1):43. doi: 10.1038/s41533-021-00255-7. NPJ Prim Care Respir Med. 2021. PMID: 34620878 Free PMC article. Review.
References
-
- Global Initiative for Chronic Obstructive Lung Disease Inc Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD, 2018. [Guidelines] 2018. [Accessed July 26, 2018]. Available from: http://goldcopd.org/
-
- Cabrera López C, Casanova Macario C, Marín Trigo JM, et al. Comparison of the 2017 and 2015 Global Initiative for Chronic Obstructive Lung Disease Reports. Impact on grouping and outcomes. Am J Respir Crit Care Med. 2018;197(4):463–469. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

