COPD treatment pathways in France: a retrospective analysis of electronic medical record data from general practitioners
- PMID: 30587961
- PMCID: PMC6305135
- DOI: 10.2147/COPD.S181224
COPD treatment pathways in France: a retrospective analysis of electronic medical record data from general practitioners
Abstract
Background: Increasing availability of therapeutic options for COPD may drive new treatment pathways. This study describes COPD treatment in France, focusing on identifying initial treatment modifications in patients with COPD who either initiated long-acting bronchodilator (LABD)-based therapy or escalated to triple therapy (long-acting muscarinic antagonist [LAMA] + long-acting β2-agonist [LABA] + inhaled corticosteroid [ICS]).
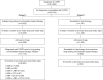
Methods: This retrospective analysis of patients with COPD in a large general practitioner database (IQVIA Longitudinal Patient Database) in France included two cohorts: Cohort 1 - new initiators of LABD-based therapy (LAMA, LABA, LAMA + LABA, LAMA + ICS, LABA + ICS or LAMA + LABA + ICS); Cohort 2 - patients escalating to triple therapy from mono- or dual-bronchodilator-based maintenance treatment. Both cohorts were indexed on the date of initiation/escalation (January 2008-December 2013), and the first treatment modification (at class level) within the 18-month post-index observational period was described. Five mutually exclusive outcomes were defined: continuous use (no modification), discontinuation (permanent [≥91 days with no restart] or temporary [≥91 days with subsequent restart]), switch, and augmentation (Cohort 1 only). Exploratory analysis of Cohort 1 explored potential drivers of treatment initiation.
Results: Overall, 5,065 patients initiated LABD-based therapy (Cohort 1), and 501 escalated to triple therapy (Cohort 2). In Cohort 1, 7.0% of patients were continuous users, 46.5% discontinued permanently, 28.5% discontinued temporarily, 2.8% augmented (added LAMA and/or LABA and/or ICS), and 15.2% switched therapy. In Cohort 2, 18.2% of patients were continuous users, 7.2% discontinued permanently, 27.9% discontinued temporarily, and 46.7% switched therapy. Exploratory analyses showed that time since COPD diagnosis was first recorded, pre-index exacerbation events, and concomitant medical conditions were potential drivers of initial maintenance treatment choices.
Conclusion: Discontinuation among new initiators of LABD-based therapy was high in France, whereas few switched or augmented treatment. In comparison, permanent discontinuation within 18 months was low in patients escalating to triple therapy.
Keywords: France; maintenance therapy; treatment modification; treatment pathways; triple therapy.
Conflict of interest statement
Disclosure WM, CCDS, GN, YP, SHL, and ASI are employees of GSK and own stocks/shares. ASI is also an unpaid faculty member at McMaster University, Hamilton, ON, Canada. RW, RJ, and TH are employees of Adelphi Real World, and GB and JD are employees of IQVIA who were contracted by GSK to conduct the study. BA has received consultant fees and/or research funds from Boehringer Ingelheim, GSK, Chiesi, Astra Zeneca, Pierre Fabre, and Roche. The authors report no other conflicts of interest in this work.
Figures




Similar articles
-
A retrospective study to assess clinical characteristics and time to initiation of open-triple therapy among patients with chronic obstructive pulmonary disease, newly established on long-acting mono- or combination therapy.Int J Chron Obstruct Pulmon Dis. 2017 Jun 21;12:1825-1836. doi: 10.2147/COPD.S129007. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28684905 Free PMC article.
-
Comparative effectiveness and safety of escalating to triple therapy versus switching to dual bronchodilators after discontinuing LABA/ICS in patients with COPD: a retrospective cohort study.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241292242. doi: 10.1177/17534666241292242. Ther Adv Respir Dis. 2024. PMID: 39491813 Free PMC article.
-
Treatment Patterns and Triple Therapy Utilization in Chinese Patients with Chronic Obstructive Pulmonary Disease: An Analysis of Real-World Data from the Yinzhou Database.Int J Chron Obstruct Pulmon Dis. 2025 Jul 29;20:2659-2670. doi: 10.2147/COPD.S499783. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40757045 Free PMC article.
-
Maintenance therapy in COPD: time to phase out ICS and switch to the new LAMA/LABA inhalers?Int J Chron Obstruct Pulmon Dis. 2017 Jun 23;12:1877-1882. doi: 10.2147/COPD.S138006. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28694698 Free PMC article. Review.
-
Triple therapy (ICS/LABA/LAMA) in COPD: time for a reappraisal.Int J Chron Obstruct Pulmon Dis. 2018 Dec 12;13:3971-3981. doi: 10.2147/COPD.S185975. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30587953 Free PMC article. Review.
Cited by
-
Real-World Effectiveness of Inhalation Therapy Among Patients With Symptomatic COPD in China: A Multicenter Prospective Study.Front Pharmacol. 2021 Sep 21;12:753653. doi: 10.3389/fphar.2021.753653. eCollection 2021. Front Pharmacol. 2021. PMID: 34621178 Free PMC article.
-
Methods to assess COPD medications adherence in healthcare databases: a systematic review.Eur Respir Rev. 2023 Sep 27;32(169):230103. doi: 10.1183/16000617.0103-2023. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37758274 Free PMC article.
-
Real-World Treatment Patterns of Multiple-Inhaler Triple Therapy Among Patients with Chronic Obstructive Pulmonary Disease in UK General Practice.Int J Chron Obstruct Pulmon Dis. 2021 May 6;16:1255-1264. doi: 10.2147/COPD.S290773. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 33986594 Free PMC article.
-
The patient journey in Chronic Obstructive Pulmonary Disease (COPD): a human factors qualitative international study to understand the needs of people living with COPD.BMC Pulm Med. 2023 Dec 13;23(1):506. doi: 10.1186/s12890-023-02796-8. BMC Pulm Med. 2023. PMID: 38093262 Free PMC article.
-
Diagnosing COPD in primary care: what has real life practice got to do with guidelines?Multidiscip Respir Med. 2019 Sep 9;14:28. doi: 10.1186/s40248-019-0191-6. eCollection 2019. Multidiscip Respir Med. 2019. PMID: 31516702 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. [Accessed November 21, 2018]. Updated 2018 Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-rev....
-
- World Health Organization [webpage on the Internet] Chronic respiratory diseases. Burden of COPD. [Accessed October 27, 2017]. Available from: http://www.who.int/respiratory/copd/burden/en/
-
- Laurendeau C, Chouaid C, Roche N, Terrioux P, Gourmelen J, Detournay B. Prise en charge et coûts de la bronchopneumopathie chronique obstructive en France en 2011. [Management and costs of chronic pulmonary obstructive disease in France in 2011] Rev Mal Respir. 2015;32(7):682–691. French. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

