Doxorubicin-loaded quaternary ammonium palmitoyl glycol chitosan polymeric nanoformulation: uptake by cells and organs
- PMID: 30587981
- PMCID: PMC6302811
- DOI: 10.2147/IJN.S176868
Doxorubicin-loaded quaternary ammonium palmitoyl glycol chitosan polymeric nanoformulation: uptake by cells and organs
Abstract
Purpose: This study was aimed to develop doxorubicin-loaded quaternary ammonium palmitoyl glycol chitosan (DOX-GCPQ) nanoformulation that could enable DOX delivery and noninvasive monitoring of drug accumulation and biodistribution at tumor site utilizing self-florescent property of doxorubicin.
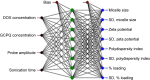
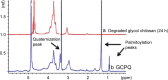
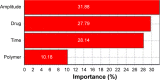
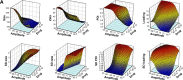
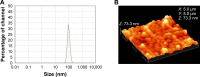
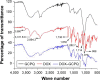
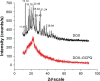
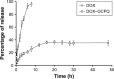
Materials and methods: DOX-GCPQ amphiphilic polymeric nanoformulations were prepared and optimized using artificial neural network (ANN) and characterized for surface morphology by atomic force microscopy, particle size with polydispersity index (PDI), and zeta potential by dynamic light scattering. Fourier transformed infrared (FTIR) and X-ray diffractometer studies were performed to examine drug polymer interaction. The ANN-optimized nanoformulation was investigated for in vitro release, cellular, tumor, and tissue uptake.
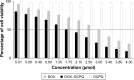
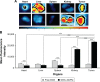
Results: The optimized DOX-GCPQ nanoformulation was anionic spherical micelles with the hydrodynamic particle size of 97.8±1.5 nm, the PDI of <0.3, the zeta potential of 28±2 mV, and the encapsulation efficiency of 80%±1.5%. Nanoformulation demonstrated a sustained release pattern over 48 h, assuming Weibull model. Fluorescence microscopy revealed higher uptake of DOX-GCPQ in human rhabdomyosarcoma (RD) cells as compared to free DOX. In vitro cytotoxicity assay indicated a significant cytotoxicity of DOX-GCPQ against RD cells as compared to DOX and blank GCPQ (P<0.05). DOX-GCPQ exhibited low IC50 (1.7±0.404 µmol) when compared to that of DOX (3.0±0.968 µmol). In skin tumor xenografts, optical imaging revealed significantly lower DOX-GCPQ in heart and liver (P<0.05) and accumulated mainly in tumor (P<0.05) as compared to other tissues.
Conclusion: The features of nanoformulation, ie, small particle size, sustained drug release, and enhanced cellular uptake, potential to target tumor passively coupled with the possibility of monitoring of tumor localization by optical imaging may make DOX-GCPQ an efficient nanotheranostic system.
Keywords: artificial neural network; biodistribution; doxorubicin; nanotheranostic; optical imaging; quaternary ammonium palmitoyl glycol chitosan.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Reduction-sensitive CD44 receptor-targeted hyaluronic acid derivative micelles for doxorubicin delivery.Int J Nanomedicine. 2018 Jul 26;13:4361-4378. doi: 10.2147/IJN.S165359. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30100720 Free PMC article.
-
Delivery of peptides to the blood and brain after oral uptake of quaternary ammonium palmitoyl glycol chitosan nanoparticles.Mol Pharm. 2012 Jun 4;9(6):1764-74. doi: 10.1021/mp300068j. Epub 2012 May 21. Mol Pharm. 2012. PMID: 22571402
-
Preparation of Modified Chitosan-based Nanoparticles for Efficient Delivery of Doxorubicin and/or Cisplatin to Breast Cancer Cells.Curr Cancer Drug Targets. 2022;22(2):133-141. doi: 10.2174/1568009622666220126100532. Curr Cancer Drug Targets. 2022. PMID: 35081892
-
Optimized DOX Drug Deliveries via Chitosan-Mediated Nanoparticles and Stimuli Responses in Cancer Chemotherapy: A Review.Molecules. 2023 Dec 20;29(1):31. doi: 10.3390/molecules29010031. Molecules. 2023. PMID: 38202616 Free PMC article. Review.
-
Single Particle Chemical Characterisation of Nanoformulations for Cargo Delivery.AAPS J. 2023 Oct 2;25(6):94. doi: 10.1208/s12248-023-00855-w. AAPS J. 2023. PMID: 37783923 Review.
Cited by
-
mAb-Functionalized Biomimetic MamC-Mediated-Magnetoliposomes as Drug Delivery Systems for Cancer Therapy.Int J Mol Sci. 2023 Sep 11;24(18):13958. doi: 10.3390/ijms241813958. Int J Mol Sci. 2023. PMID: 37762260 Free PMC article.
-
Design development and optimisation of multifunctional Doxorubicin-loaded Indocynanine Green proniosomal gel derived niosomes for tumour management.Sci Rep. 2023 Jan 30;13(1):1697. doi: 10.1038/s41598-023-28891-8. Sci Rep. 2023. PMID: 36717736 Free PMC article.
-
Soy protein isolate-carboxymethyl cellulose conjugates with pH sensitivity for sustained avermectin release.R Soc Open Sci. 2019 Jul 17;6(7):190685. doi: 10.1098/rsos.190685. eCollection 2019 Jul. R Soc Open Sci. 2019. PMID: 31417761 Free PMC article.
-
Nanoformulation Design Including MamC-Mediated Biomimetic Nanoparticles Allows the Simultaneous Application of Targeted Drug Delivery and Magnetic Hyperthermia.Polymers (Basel). 2020 Aug 15;12(8):1832. doi: 10.3390/polym12081832. Polymers (Basel). 2020. PMID: 32824256 Free PMC article.
-
Internalization and effects on cellular ultrastructure of nickel nanoparticles in rat kidneys.Int J Nanomedicine. 2019 May 29;14:3995-4005. doi: 10.2147/IJN.S200909. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31213811 Free PMC article.
References
-
- Lee VH, Robinson JR. Controlled Drug Delivery: Fundamentals and Applications. New York: Marcel Dekker; 1987.
-
- Deepa K, Singha S, Panda T. Doxorubicin nanoconjugates. J Nanosci Nanotechnol. 2014;14(1):892–904. - PubMed
-
- Danesi R, Fogli S, Gennari A, Conte P, Del Tacca M. Pharmacokinetic-pharmacodynamic relationships of the anthracycline anticancer drugs. Clin Pharmacokinet. 2002;41(6):431–444. - PubMed
-
- Outomuro D, Grana DR, Azzato F, Milei J. Adriamycin-induced myocardial toxicity: new solutions for an old problem? Int J Cardiol. 2007;117(1):6–15. - PubMed
-
- Hilger RA, Richly H, Grubert M, et al. Pharmacokinetics (PK) of a liposomal encapsulated fraction containing doxorubicin and of doxorubicin released from the liposomal capsule after intravenous infusion of Caelyx/Doxil. Int J Clin Pharmacol Ther. 2005;43(12):588–589. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

