Doxorubicin and indocyanine green loaded superparamagnetic iron oxide nanoparticles with PEGylated phospholipid coating for magnetic resonance with fluorescence imaging and chemotherapy of glioma
- PMID: 30587988
- PMCID: PMC6304244
- DOI: 10.2147/IJN.S173954
Doxorubicin and indocyanine green loaded superparamagnetic iron oxide nanoparticles with PEGylated phospholipid coating for magnetic resonance with fluorescence imaging and chemotherapy of glioma
Abstract
Background: Glioma represents the most common malignant brain tumor. Outcomes of surgical resection are often unsatisfactory due to low sensitivity or resolution of imaging methods. Moreover, the use of traditional chemotherapeutics, such as doxorubicin (DOX), is limited due to their low blood-brain barrier (BBB) permeability. Recently, the development of nanotechnology could overcome these obstacles.
Materials and methods: Hydrophobic superparamagnetic iron oxide nanoparticles (SPIO NPs) were prepared with the use of thermal decomposition method. They were coated with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG 2000) and DOX using a thin-film hydration method followed by loading of indocyanine green (ICG) into the phospholipid layers. Details regarding the characteristics of NPs were determined. The in vitro biocompatibility and antitumor efficacy were established with the use of MTT assay. In vivo fluorescence and magnetic resonance (MR) imaging were used to evaluate BBB penetration and accumulation of NPs at the tumor site. Antitumor efficacy was evaluated using measures of tumor size, median survival times, body weights, and H&E staining.
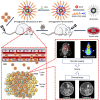
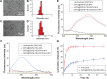
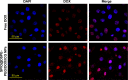
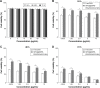
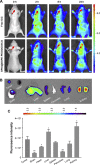
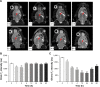
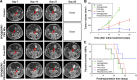
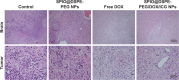
Results: The multifunctional NPs generated had an average diameter of 22.9 nm, a zeta potential of -38.19 mV, and were capable of providing a sustained release of DOX. In vitro experiments demonstrated that the SPIO@DSPE-PEG/DOX/ICG NPs effectively enhanced cellular uptake of DOX as compared with that of free DOX. In vivo fluorescence and MR imaging revealed that the NPs not only effectively crossed the BBB but selectively accumulated at the tumor site. Meanwhile, among all groups studied, C6 glioma-bearing rats treated with the NPs exhibited the maximal degree of therapeutic efficacy, including smallest tumor volume, lowest body weight loss, and longest survival times, with no obvious side effects.
Conclusion: These results suggest that the SPIO@DSPE-PEG/DOX/ICG NPs can not only function as a nanoprobe for MR and fluorescence bimodal imaging, but also as a vehicle to deliver chemotherapeutic drugs to the tumor site, to achieve the theranostic treatment of glioma.
Keywords: BBB; MR imaging; SPIO NPs; chemotherapy; fluorescence imaging.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Indocyanine green loaded SPIO nanoparticles with phospholipid-PEG coating for dual-modal imaging and photothermal therapy.Biomaterials. 2013 Oct;34(31):7706-14. doi: 10.1016/j.biomaterials.2013.07.007. Epub 2013 Jul 17. Biomaterials. 2013. PMID: 23871538
-
ROS-Responsive Nanoprobes for Bimodal Imaging-Guided Cancer Targeted Combinatorial Therapy.Int J Nanomedicine. 2024 Aug 7;19:8071-8090. doi: 10.2147/IJN.S467512. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39130685 Free PMC article.
-
Doxorubicin-modified magnetic nanoparticles as a drug delivery system for magnetic resonance imaging-monitoring magnet-enhancing tumor chemotherapy.Int J Nanomedicine. 2016 May 12;11:2021-37. doi: 10.2147/IJN.S94139. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27274233 Free PMC article.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Doxorubicin-loaded poly(ethylene oxide)-trimellitic anhydride chloride-folate superparamagnetic iron oxide nanoparticles.2010 Jul 16 [updated 2010 Sep 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jul 16 [updated 2010 Sep 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20945566 Free Books & Documents. Review.
Cited by
-
Non-invasive sensitive brain tumor detection using dual-modality bioimaging nanoprobe.Nanotechnology. 2019 Jul 5;30(27):275101. doi: 10.1088/1361-6528/ab0e9c. Epub 2019 Mar 11. Nanotechnology. 2019. PMID: 30856613 Free PMC article.
-
Use of Super Paramagnetic Iron Oxide Nanoparticles as Drug Carriers in Brain and Ear: State of the Art and Challenges.Brain Sci. 2021 Mar 11;11(3):358. doi: 10.3390/brainsci11030358. Brain Sci. 2021. PMID: 33799690 Free PMC article. Review.
-
Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin.J Nanobiotechnology. 2021 Sep 26;19(1):289. doi: 10.1186/s12951-021-01048-3. J Nanobiotechnology. 2021. PMID: 34565383 Free PMC article.
-
Magnetic Iron Oxide Nanoparticles for Biomedical Applications.Curr Opin Biomed Eng. 2021 Dec;20:100330. doi: 10.1016/j.cobme.2021.100330. Epub 2021 Aug 17. Curr Opin Biomed Eng. 2021. PMID: 35211662 Free PMC article.
-
Nanoparticle-based materials in anticancer drug delivery: Current and future prospects.Heliyon. 2023 Oct 20;9(11):e21227. doi: 10.1016/j.heliyon.2023.e21227. eCollection 2023 Nov. Heliyon. 2023. PMID: 37954330 Free PMC article. Review.
References
-
- Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. - PubMed
-
- Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6(11):648–657. - PubMed
-
- Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012;26(4):825–853. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

