Cytokine production capabilities of human primary monocyte-derived macrophages from patients with diabetes mellitus type 2 with and without diabetic peripheral neuropathy
- PMID: 30588081
- PMCID: PMC6305162
- DOI: 10.2147/JPR.S186372
Cytokine production capabilities of human primary monocyte-derived macrophages from patients with diabetes mellitus type 2 with and without diabetic peripheral neuropathy
Abstract
Introduction: Monocytes from patients with diabetes mellitus type 2 (DM2) are dysfunctional, persistently primed, and prone to a proinflammatory phenotype. This may alter the phenotype of their differentiation to macrophages and result in diabetic peripheral neuropathy (DPN), nerve damage, nerve sensitization, and chronic pain. We have previously demonstrated that CD163 is a molecule that promotes an anti-inflammatory cellular phenotype in human primary macrophages, but this has not been proven in macrophages from patients with DM2 or DPN. Thus, we hypothesize that macrophages from patients with DM2 or DPN display an altered proinflammatory functional phenotype related to cytokine production and that the induction of CD163 expression will promote a more homeostatic phenotype by reducing their proinflammatory responsiveness.
Patients and methods: We tested these hypotheses in vitro using blood monocyte-derived macrophages from healthy subjects and patients with DM2 with and without DPN. Cells were incubated in the presence or the absence of 5 µg/mL of lipopolysaccharide (LPS). The concentrations of interleukin-10, interleukin-6, tumor necrosis factor-alpha (TNF-α), TGF-β, and monocyte chemoattractant protein-1 (MCP-1) were measured using ELISA assays. Macrophages were transfected with an empty vector plasmid or a plasmid containing the CD163 gene using mannosylated polyethylenimine nanoparticles.
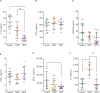
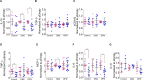
Results: Our results show that nonstimulated DM2 or DPN macrophages have a constitutive primed proinflammatory state and display a deficient production of proinflammatory cytokines upon a proinflammatory challenge when compared to healthy macrophages. CD163 induction produced an anti-inflammatory phenotype in the healthy control group, and this effect was partial in DM2 or DPN macrophages.
Conclusion: Our results suggest that diabetic macrophages adopt a complex phenotype that is only partially reversed by CD163 induction. Future experiments are focused on elucidating this differential responsiveness between healthy and diabetic macrophages.
Keywords: CD163; LPS; primary human macrophages; transfection.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

