Efficacy and safety of primary surgery with postoperative radiotherapy in head and neck mucosal melanoma: a single-arm Phase II study
- PMID: 30588103
- PMCID: PMC6298389
- DOI: 10.2147/CMAR.S185017
Efficacy and safety of primary surgery with postoperative radiotherapy in head and neck mucosal melanoma: a single-arm Phase II study
Abstract
Background: There still remains no well-established treatment strategy for head and neck mucosal melanoma (HNMM). We aim to evaluate the effectiveness and safety of primary surgery with postoperative radiotherapy for this disease.
Patients and methods: A single-arm, Phase II clinical trial was conducted at Sun Yat-Sen University Cancer Center. Patients with nonmetastatic, histologically proven HNMM were prospectively enrolled. Patients received primary surgery followed by intensity-modulated radiotherapy with an equivalent dose at 2 Gy per fraction of 65-70 Gy to CTV1 (high-risk regions including tumor bed) and 50-55 Gy to CTV2 (low-risk regions). Additional use of adjuvant chemotherapy (AC) depended on consultation from a multidisciplinary team. This trial is registered with ClinicalTrials.gov, number NCT03138642.
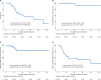
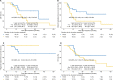
Results: A total of 33 patients were enrolled and analyzed between July 2010 and November 2016. There were 18 (54.5%) patients with T3 disease and 15 (45.5%) patients with T4a disease. The median age at diagnosis was 58 years (range 27-83 years), and 61% of the cohort were males. The overall median follow-up duration was 25.3 months (range 5.3-67.1 months). The 3-year overall survival (OS), local relapse-free survival (LRFS), regional relapse-free survival (RRFS), and distant metastasis-free survival (DMFS) rates were 44.4, 91.7, 78.1, and 41.7%, respectively. Patients with T4a disease showed significantly inferior OS (P=0.049) and DMFS (P=0.040) than those with T3 disease. Prophylactic neck radiation (PNR) was nearly associated with superior RRFS (P=0.078). However, there was no significant difference in OS, LRFS, RRFS, and DMFS for patients treated with or without AC (P>0.05 for all). Toxicities were generally mild to moderate.
Conclusion: Primary surgery with postoperative radiotherapy yielded excellent local control and acceptable toxicity profile for HNMM. Nevertheless, high rates of distant metastases resulted in limited survival.
Keywords: efficacy; head and neck mucosal melanoma; postoperative radiotherapy; primary surgery; safety.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Gutman M, Inbar M, Chaitchik S, et al. Malignant melanoma of the mucous membranes. Eur J Surg Oncol. 1992;18(4):307–312. - PubMed
-
- Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28(2):96–102. - PubMed
-
- Lourenço SV, A MS, Sotto MN, et al. Primary oral mucosal melanoma: a series of 35 new cases from South America. Am J Dermatopathol. 2009;31(4):323–330. - PubMed
-
- Ross MI, Henderson MA. Mucosal melanoma. In: Balch CM, Houghton AN, Sober AJ, et al., editors. Cutaneous Melanoma. 5th ed. St Louis, MO: Quality Medical Publishing; 2009. pp. 337–350.
Associated data
LinkOut - more resources
Full Text Sources
Medical

