Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways
- PMID: 30588110
- PMCID: PMC6304079
- DOI: 10.2147/CMAR.S186696
Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways
Abstract
Background/purpose: Pristimerin (Pris) is triterpenoid compound with many biological effects. Until now, nothing is known about its effect on doxorubicin (DOX)-induced cardiotoxicity. Hence, this study investigated the impact of Pris on DOX-induced cardiotoxic effects.
Materials and methods: Rats were treated with Pris 1 week before and 2 weeks contaminant with repeated DOX injection. Afterwards, electrocardiography (ECG), biochemical, histopathological, PCR, and Western blot assessments were performed.
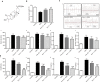
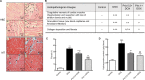
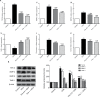
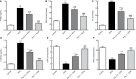
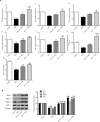
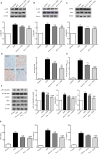
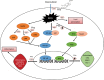
Results: Pris effectively alleviated DOX-induced deleterious cardiac damage. It inhibited DOX-induced ECG abnormities as well as DOX-induced elevation of serum indices of cardiotoxicity. The histopathological cardiac lesions and fibrosis were remarkably improved in Pris-treated animals. Pris reduced hydroxyproline content and attenuated the mRNA and protein expression of the pro-fibrogenic genes. The antioxidant activity of Pris was prominent through the amelioration of oxidative stress parameters and enhancement of antioxidants. Furthermore, Pris enhanced the activation of nuclear factor-erythroid 2 related factor 2 (Nrf2) signaling pathway as it increased the mRNA and protein expression of Nrf2 and Nrf2-dependent antioxidant genes (GCL, NQO1, HO-1). Additionally, the anti-inflammatory effect of Pris was obvious through the inhibition of mitogen activated protein kinase (MAPK)/nuclear factor kappa-B (NF-kB) signaling and subsequent inhibition of inflammatory mediators.
Conclusion: This study provides evidence of the cardioprotective activity of Pris which is related to the modulation of Nrf2 and MAPK/NF-kB signaling pathways.
Keywords: MAPK/NF-kB; Nrf2; cardiotoxicity; doxorubicin; pristimerin.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Pristimerin as a Novel Hepatoprotective Agent Against Experimental Autoimmune Hepatitis.Front Pharmacol. 2018 Mar 28;9:292. doi: 10.3389/fphar.2018.00292. eCollection 2018. Front Pharmacol. 2018. PMID: 29643811 Free PMC article.
-
α-Bisabolol, a Dietary Sesquiterpene, Attenuates Doxorubicin-Induced Acute Cardiotoxicity in Rats by Inhibiting Cellular Signaling Pathways, Nrf2/Keap-1/HO-1, Akt/mTOR/GSK-3β, NF-κB/p38/MAPK, and NLRP3 Inflammasomes Regulating Oxidative Stress and Inflammatory Cascades.Int J Mol Sci. 2023 Sep 13;24(18):14013. doi: 10.3390/ijms241814013. Int J Mol Sci. 2023. PMID: 37762315 Free PMC article.
-
Irbesartan suppresses cardiac toxicity induced by doxorubicin via regulating the p38-MAPK/NF-κB and TGF-β1 pathways.Naunyn Schmiedebergs Arch Pharmacol. 2019 Jun;392(6):647-658. doi: 10.1007/s00210-019-01624-3. Epub 2019 Feb 7. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 30734091
-
Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling.Biomed Pharmacother. 2020 Feb;122:109547. doi: 10.1016/j.biopha.2019.109547. Epub 2019 Dec 30. Biomed Pharmacother. 2020. PMID: 31918264
-
Natural compounds against doxorubicin-induced cardiotoxicity: A review on the involvement of Nrf2/ARE signaling pathway.Phytother Res. 2021 Mar;35(3):1163-1175. doi: 10.1002/ptr.6882. Epub 2020 Sep 28. Phytother Res. 2021. PMID: 32985744 Review.
Cited by
-
α-Bisabolol Attenuates Doxorubicin Induced Renal Toxicity by Modulating NF-κB/MAPK Signaling and Caspase-Dependent Apoptosis in Rats.Int J Mol Sci. 2022 Sep 10;23(18):10528. doi: 10.3390/ijms231810528. Int J Mol Sci. 2022. PMID: 36142441 Free PMC article.
-
Targeting Oxidative Stress, NLRP3 Inflammasome, and Autophagy by Fraxetin to Combat Doxorubicin-Induced Cardiotoxicity.Pharmaceuticals (Basel). 2021 Nov 20;14(11):1188. doi: 10.3390/ph14111188. Pharmaceuticals (Basel). 2021. PMID: 34832970 Free PMC article.
-
TFEB-NF-κB inflammatory signaling axis: a novel therapeutic pathway of Dihydrotanshinone I in doxorubicin-induced cardiotoxicity.J Exp Clin Cancer Res. 2020 May 24;39(1):93. doi: 10.1186/s13046-020-01595-x. J Exp Clin Cancer Res. 2020. PMID: 32448281 Free PMC article.
-
Pristimerin inhibits neuronal inflammation and protects cognitive function in mice with sepsis-induced brain injuries by regulating PI3K/Akt signalling.Pharm Biol. 2021 Dec;59(1):1351-1358. doi: 10.1080/13880209.2021.1981399. Pharm Biol. 2021. Retraction in: Pharm Biol. 2024 Dec;62(1):269. doi: 10.1080/13880209.2024.2323353. PMID: 34590530 Free PMC article. Retracted.
-
NLRP3 inflammasome as a therapeutic target in doxorubicin-induced cardiotoxicity: role of phytochemicals.Front Pharmacol. 2025 Apr 17;16:1567312. doi: 10.3389/fphar.2025.1567312. eCollection 2025. Front Pharmacol. 2025. PMID: 40313623 Free PMC article. Review.
References
-
- Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34(1):106–135. - PubMed
-
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213–1225. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

