Measuring prevalence and incidence of chronic conditions in claims and electronic health record databases
- PMID: 30588119
- PMCID: PMC6301730
- DOI: 10.2147/CLEP.S181242
Measuring prevalence and incidence of chronic conditions in claims and electronic health record databases
Abstract
Background: Health care databases are natural sources for estimating prevalence and incidence of chronic conditions, but substantial variation in estimates limits their interpretability and utility. We evaluated the effects of design choices when estimating prevalence and incidence in claims and electronic health record databases.
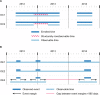
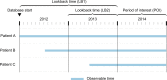
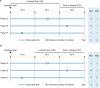
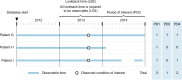
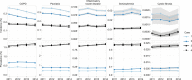
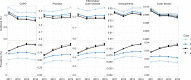
Methods: Prevalence and incidence for five chronic diseases at increasing levels of expected frequencies, from cystic fibrosis to COPD, were estimated in the Clinical Practice Research Datalink (CPRD) and MarketScan databases from 2011 to 2014. Estimates were compared using different definitions of lookback time and contributed person-time.
Results: Variation in lookback time substantially affected estimates. In 2014, for CPRD, use of an all-time vs a 1-year lookback window resulted in 4.3-8.3 times higher prevalence (depending on disease), reducing incidence by 1.9-3.3 times. All-time lookback resulted in strong temporal trends. COPD prevalence between 2011 and 2014 in MarketScan increased by 25% with an all-time lookback but stayed relatively constant with a 1-year lookback. Varying observability did not substantially affect estimates.
Conclusion: This framework draws attention to the underrecognized potential for widely varying incidence and prevalence estimates, with implications for care planning and drug development. Though prevalence and incidence are seemingly straightforward concepts, careful consideration of methodology is required to obtain meaningful estimates from health care databases.
Keywords: cross-sectional studies; epidemiologic methods; epidemiological monitoring; epidemiology; incidence; pharmacoepidemiology; prevalence; prevalence studies; secondary databases; sentinel surveillance.
Conflict of interest statement
Disclosure Jeremy A Rassen is an employee of and has an ownership interest in Aetion, Inc, a technology company that provides analytic software and services to the health care industry. Dorothee B Bartels is an employee of Boehringer Ingelheim, which is a customer of Aetion, Inc. Sebastian Schneeweiss is a consultant to World Health Information Science Consultants (WHISCON), LLC, and to Aetion, Inc, in which he also owns equity. He is the principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Bayer, Genentech, and Boehringer Ingelheim. Amanda R Patrick is an employee of and has ownership in Aetion, Inc. At the time of writing, William Murk was an employee of and had ownership in Aetion, Inc, in which he has an ownership interest. The authors report no other conflicts of interest in this work.
Figures

References
-
- Rothman KJ, Greenland S1, Lash TL. Modern Epidemiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008.
-
- Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. - PubMed
-
- Franklin JM, Schneeweiss S. When and How Can Real World Data Analyses Substitute for Randomized Controlled Trials? Clin Pharmacol Ther. 2017;102(6):924–933. - PubMed
-
- Lin KJ, Schneeweiss S. Considerations for the analysis of longitudinal electronic health records linked to claims data to study the effectiveness and safety of drugs. Clin Pharmacol Ther. 2016;100(2):147–159. - PubMed
LinkOut - more resources
Full Text Sources

