Observational studies of treatment effectiveness: worthwhile or worthless?
- PMID: 30588122
- PMCID: PMC6302806
- DOI: 10.2147/CLEP.S178723
Observational studies of treatment effectiveness: worthwhile or worthless?
Abstract
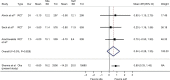
Observational studies which evaluate effectiveness are often viewed with skepticism owing to the fact that patients are not randomized to treatment, meaning that results are more prone to bias. Therefore, randomized controlled trials remain the gold standard for evaluating treatment effectiveness. However, it is not always possible to conduct randomized trials. This may be due to financial constraints, for example, in identifying funding for a randomized trial for medicines that have already gained market authorization. There can also be challenges with recruitment, for example, of people with rare conditions or in hard-to-reach population subgroups. This is why observational studies are still needed. In this manuscript, we discuss how researchers can mitigate the risk of bias in the most common type of observational study design for evaluation of treatment effectiveness, the cohort study. We outline some key issues that warrant careful consideration at the outset when the question is being developed and the cohort study is being designed. We focus our discussion on the importance of deciding when to start follow-up in a study, choosing a comparator, managing confounding and measuring outcomes. We also illustrate the application of these considerations in a more detailed case study based on an examination of comparative effectiveness of two antidiabetic treatments using data collected during routine clinical practice.
Keywords: diabetes mellitus; effectiveness; epidemiology; public health; therapeutics.
Conflict of interest statement
Disclosure All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. MS, IN and IP report grants from Novo Nordisk A/S during the conduct of the study. The views expressed are those of the authors and not necessarily those of Novo Nordisk A/S. The authors (MS, IN and IP) report no other conflicts of interest in this work.
Figures
References
-
- Greenland S, Mansournia MA. Limitations of individual causal models, causal graphs, and ignorability assumptions, as illustrated by random confounding and design unfaithfulness. Eur J Epidemiol. 2015;30(10):1101–1110. - PubMed
-
- Pocock SJ, Elbourne DR. Randomized trials or observational tribulations? N Engl J Med. 2000;342(25):1907–1909. - PubMed
-
- Freemantle N, Marston L, Walters K, et al. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ. 2013;347:f6409. - PubMed
LinkOut - more resources
Full Text Sources

