Phenotypic Alteration of Hepatocytes in Non-Alcoholic Fatty Liver Disease
- PMID: 30588181
- PMCID: PMC6299410
- DOI: 10.7150/ijms.27953
Phenotypic Alteration of Hepatocytes in Non-Alcoholic Fatty Liver Disease
Abstract
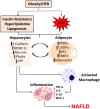
Non-Alcoholic Fatty Liver Disease (NAFLD) has been recognized as the most common liver disorder in developed countries. NAFLD progresses from fat accumulation in hepatocytes to steatohepatitis to further stages of fibrosis and cirrhosis. Simple steatosis, i.e. fat deposition in the liver, is considered benign and gives way to non-alcoholic steatohepatitis (NASH) with a higher probability of progressing to cirrhosis, and liver-related mortality. Evidence has been found that this progression has been associated with marked alterations in hepatocyte histology and a shift in marker expression of healthy hepatocytes including increased expression of peroxisome proliferator-activated receptor gamma (PPARγ), adipocyte protein (aP2), CD36, interleukin-6 (IL-6), interleukin-18 (IL-18) and adiponectin. This progression shares much in common with the obesity phenotype, which involves a transformation of adipocytes from small, healthy cells to large, dysfunctional ones that contribute to redox imbalance and the progression of metabolic syndrome. Further, activation of Src/ERK signaling via the sodium potassium adenosine triphosphatase (Na/K-ATPase) α-1 subunit in impaired hepatocytes may contribute to redox imbalance, exacerbating the progression of NAFLD. This review hypothesizes that an adipogenic transformation of hepatocytes propagates redox imbalance and that the processes occurring in adipogenesis become activated in fat-laden hepatocytes in liver, thereby driving progression to NAFLD. Further, this review discusses therapeutic interventions to reverse NAFLD including the thiazolidinediones (TZDs) and a variety of antioxidant species. The peptide, pNaKtide, which is an antagonist of Na/K-ATPase signaling, is also proposed as a potential pharmacologic option for reducing reactive oxygen species (ROS) and reversing NAFLD by inhibiting the Na/K-ATPase-modulated ROS amplification loop.
Keywords: Hepatocytes; Non-Alcoholic Fatty Liver Disease.
Conflict of interest statement
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

