Anifrolumab effects on rash and arthritis: impact of the type I interferon gene signature in the phase IIb MUSE study in patients with systemic lupus erythematosus
- PMID: 30588322
- PMCID: PMC6280909
- DOI: 10.1136/lupus-2018-000284
Anifrolumab effects on rash and arthritis: impact of the type I interferon gene signature in the phase IIb MUSE study in patients with systemic lupus erythematosus
Abstract
Objective: This post hoc analysis compared anifrolumab 300 mg every 4 weeks with placebo on rash and arthritis measures with different stringency in patients with moderate to severe SLE (phase IIb; MUSE; NCT01438489). Subgroups were analysed by type I interferon gene signature (IFNGS test-high or test-low).
Methods: Rash was measured with the SLE Disease Activity Index 2000 (SLEDAI-2K), British Isles Lupus Assessment Group (BILAG) Index and modified Cutaneous Lupus Erythematosus Disease Area and Severity Index (mCLASI). Arthritis was evaluated using SLEDAI-2K, BILAG and swollen and tender joint counts. Outcomes were measured at week 52.
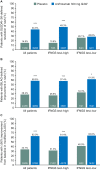
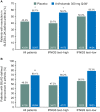
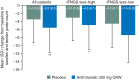
Results: More anifrolumab-treated patients demonstrated resolution of rash by SLEDAI-2K versus placebo: 39/88 (44.3%) versus 13/88 (14.8%), OR (90% CI) 4.56 (2.48 to 8.39), p<0.001; improvement of BILAG: 48/82 (58.5%) versus 24/85 (28.2%), OR (90% CI) 3.59 (2.08 to 6.19), p<0.001; and ≥50% improvement by mCLASI: 57/92 (62.0%) versus 30/89 (33.7%), OR (90% CI) 3.31 (1.97 to 5.55), p<0.001. More anifrolumab-treated patients had improved arthritis by SLEDAI-2K versus placebo: 55/97 (56.7%) versus 42/99 (42.4%), OR (90% CI) 1.88 (1.16 to 3.04), p=0.032; and BILAG: 65/94 (69.1%) versus 47/95 (49.5%), OR (90% CI) 2.47 (1.48 to 4.12), p=0.003; and mean (SD) swollen and tender joint reductions: -5.5 (6.3) versus -3.4 (5.9), p=0.004. Comparable results were demonstrated in IFNGS test-high patients (n=151). In IFNGS test-low patients (n=50), substantial numerical differences in partial rash and arthritis responses were observed in anifrolumab-treated patients versus placebo, with statistical significance only for rash by BILAG in this small population.
Conclusions: Anifrolumab treatment was associated with improvements versus placebo in specific SLE features of arthritis and rash using measures of different stringency. Although driven by robust data in the prevalent IFNGS test-high population, further evaluation in IFNGS test-low patients is warranted.
Keywords: Arthritis; Interferon; Treatment.
Conflict of interest statement
Competing interests: JTM has received grants and personal fees from AstraZeneca during the conduct of the study. She has also received grants from GSK, BMS, Xencor and Exagen; consulting fees from GSK, BMS, EMD Serono, Calgene, UCB, RemeGen, Exagen, Lilly, Amgen, Janssen, Sanofi, Anthera and Neovacs; and fees for participating in data safety monitoring boards from Immunopharma and Takeda, outside the submitted work. RF has been a consultant and investigator for MedImmune/AstraZeneca and has participated in an advisory board for MedImmune/AstraZeneca. VPW has been a consultant for MedImmune and has received a grant from AstraZeneca. The University of Pennsylvania owns copyright over the CLASI. MK reports no conflicts of interest. JD, LW and GI are employees of Viela Bio. RT is an employee of AstraZeneca. JD, GI, LW and RT own stock with AstraZeneca.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
