Skeletal muscle stem cells in comfort and stress
- PMID: 30588332
- PMCID: PMC6303387
- DOI: 10.1038/s41536-018-0062-3
Skeletal muscle stem cells in comfort and stress
Abstract
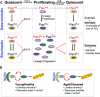
Investigations on developmental and regenerative myogenesis have led to major advances in decrypting stem cell properties and potential, as well as their interactions within the evolving niche. As a consequence, regenerative myogenesis has provided a forum to investigate intrinsic regulators of stem cell properties as well as extrinsic factors, including stromal cells, during normal growth and following injury and disease. Here we review some of the latest advances in the field that have exposed fundamental processes including regulation of stress following trauma and ageing, senescence, DNA damage control and modes of symmetric and asymmetric cell divisions. Recent studies have begun to explore the nature of the niche that is distinct in different muscle groups, and that is altered from prenatal to postnatal stages, and during ageing. We also discuss heterogeneities among muscle stem cells and how distinct properties within the quiescent and proliferating cell states might impact on homoeostasis and regeneration. Interestingly, cellular quiescence, which was thought to be a passive cell state, is regulated by multiple mechanisms, many of which are deregulated in various contexts including ageing. These and other factors including metabolic activity and genetic background can impact on the efficiency of muscle regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

