A novel nitroalkene-α-tocopherol analogue inhibits inflammation and ameliorates atherosclerosis in Apo E knockout mice
- PMID: 30588602
- PMCID: PMC6393233
- DOI: 10.1111/bph.14561
A novel nitroalkene-α-tocopherol analogue inhibits inflammation and ameliorates atherosclerosis in Apo E knockout mice
Abstract
Background and purpose: Atherosclerosis is characterized by chronic low-grade inflammation with concomitant lipid accumulation in the arterial wall. Anti-inflammatory and anti-atherogenic properties have been described for a novel class of endogenous nitroalkenes (nitrated-unsaturated fatty acids), formed during inflammation and digestion/absorption processes. The lipid-associated antioxidant α-tocopherol is transported systemically by LDL particles including to the atheroma lesions. To capitalize on the overlapping and complementary salutary properties of endogenous nitroalkenes and α-tocopherol, we designed and synthesized a novel nitroalkene-α-tocopherol analogue (NATOH) to address chronic inflammation and atherosclerosis, particularly at the lesion sites.
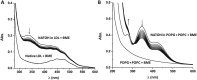
Experimental approach: We synthesized NATOH, determined its electrophilicity and antioxidant capacity and studied its effects over pro-inflammatory and cytoprotective pathways in macrophages in vitro. Moreover, we demonstrated its incorporation into lipoproteins and tissue both in vitro and in vivo, and determined its effect on atherosclerosis and inflammatory responses in vivo using the Apo E knockout mice model.
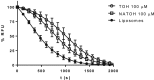
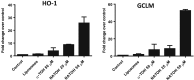
Key results: NATOH exhibited similar antioxidant capacity to α-tocopherol and, due to the presence of the nitroalkenyl group, like endogenous nitroalkenes, it exerted electrophilic reactivity. NATOH was incorporated in vivo into the VLDL/LDL lipoproteins particles to reach the atheroma lesions. Furthermore, oral administration of NATOH down-regulated NF-κB-dependent expression of pro-inflammatory markers (including IL-1β and adhesion molecules) and ameliorated atherosclerosis in Apo E knockout mice.
Conclusions and implications: In toto, the data demonstrate a novel pharmacological strategy for the prevention of atherosclerosis based on a creative, natural and safe drug delivery system of a non-conventional anti-inflammatory compound (NATOH) with significant potential for clinical application.
© 2018 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
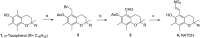







Similar articles
-
Electrophilic nitroalkene-tocopherol derivatives: synthesis, physicochemical characterization and evaluation of anti-inflammatory signaling responses.Sci Rep. 2018 Aug 24;8(1):12784. doi: 10.1038/s41598-018-31218-7. Sci Rep. 2018. PMID: 30143727 Free PMC article.
-
A thioredoxin-mimetic peptide exerts potent anti-inflammatory, antioxidant, and atheroprotective effects in ApoE2.Ki mice fed high fat diet.Cardiovasc Res. 2019 Feb 1;115(2):292-301. doi: 10.1093/cvr/cvy183. Cardiovasc Res. 2019. PMID: 30010817
-
The effect of tocopheryl phosphates (TPM) on the development of atherosclerosis in apolipoprotein-E deficient mice.Clin Exp Pharmacol Physiol. 2017 Dec;44 Suppl 1:107-116. doi: 10.1111/1440-1681.12821. Epub 2017 Sep 18. Clin Exp Pharmacol Physiol. 2017. PMID: 28744946
-
Antioxidant and Anti-Inflammatory Effects of Bioactive Compounds in Atherosclerosis.Int J Mol Sci. 2025 Feb 6;26(3):1379. doi: 10.3390/ijms26031379. Int J Mol Sci. 2025. PMID: 39941147 Free PMC article. Review.
-
Tocopherols in the Prevention and Treatment of Atherosclerosis and Related Cardiovascular Disease.Clin Cardiol. 2015 Sep;38(9):570-6. doi: 10.1002/clc.22422. Epub 2015 Aug 14. Clin Cardiol. 2015. PMID: 26272221 Free PMC article. Review.
Cited by
-
A Nitroalkene Benzoic Acid Derivative Targets Reactive Microglia and Prolongs Survival in an Inherited Model of ALS via NF-κB Inhibition.Neurotherapeutics. 2021 Jan;18(1):309-325. doi: 10.1007/s13311-020-00953-z. Epub 2020 Oct 28. Neurotherapeutics. 2021. PMID: 33118131 Free PMC article.
-
A nitroalkene derivative of salicylate, SANA, induces creatine-dependent thermogenesis and promotes weight loss.Nat Metab. 2025 Aug;7(8):1550-1569. doi: 10.1038/s42255-025-01311-z. Epub 2025 Jun 17. Nat Metab. 2025. PMID: 40527924 Free PMC article. Clinical Trial.
-
A derivative of the ancient drug salicylate for obesity treatment.Nat Metab. 2025 Aug;7(8):1509-1510. doi: 10.1038/s42255-025-01312-y. Nat Metab. 2025. PMID: 40588540 No abstract available.
-
A nitroalkene derivative of salicylate alleviates diet-induced obesity by activating creatine metabolism and non-shivering thermogenesis.Res Sq [Preprint]. 2023 Jul 12:rs.3.rs-3101395. doi: 10.21203/rs.3.rs-3101395/v1. Res Sq. 2023. Update in: Nat Metab. 2025 Aug;7(8):1550-1569. doi: 10.1038/s42255-025-01311-z. PMID: 37502859 Free PMC article. Updated. Preprint.
-
A novel nitroalkene vitamin E analogue inhibits the NLRP3 inflammasome and protects against inflammation and glucose intolerance triggered by obesity.Redox Biol. 2021 Feb;39:101833. doi: 10.1016/j.redox.2020.101833. Epub 2020 Dec 15. Redox Biol. 2021. PMID: 33352465 Free PMC article.
References
-
- Batthyany C, Santos CX, Botti H, Cervenansky C, Radi R, Augusto O et al (2000). Direct evidence for apo B‐100‐mediated copper reduction: studies with purified apo B‐100 and detection of tryptophanyl radicals. Arch Biochem Biophys 384: 335–340. - PubMed
-
- Botti H, Batthyany C, Trostchansky A, Radi R, Freeman BA, Rubbo H (2004). Peroxynitrite‐mediated α‐tocopherol oxidation in low‐density lipoprotein: a mechanistic approach. Free Radic Biol Med 36: 152–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

