Prion neurotoxicity
- PMID: 30588688
- PMCID: PMC6894960
- DOI: 10.1111/bpa.12694
Prion neurotoxicity
Abstract
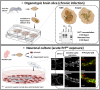
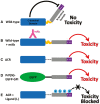
Although the mechanisms underlying prion propagation and infectivity are now well established, the processes accounting for prion toxicity and pathogenesis have remained mysterious. These processes are of enormous clinical relevance as they hold the key to identification of new molecular targets for therapeutic intervention. In this review, we will discuss two broad areas of investigation relevant to understanding prion neurotoxicity. The first is the use of in vitro experimental systems that model key events in prion pathogenesis. In this context, we will describe a hippocampal neuronal culture system we developed that reproduces the earliest pathological alterations in synaptic morphology and function in response to PrPSc . This system has allowed us to define a core synaptotoxic signaling pathway involving the activation of NMDA and AMPA receptors, stimulation of p38 MAPK phosphorylation and collapse of the actin cytoskeleton in dendritic spines. The second area concerns a striking and unexpected phenomenon in which certain structural manipulations of the PrPC molecule itself, including introduction of N-terminal deletion mutations or binding of antibodies to C-terminal epitopes, unleash powerful toxic effects in cultured cells and transgenic mice. We will describe our studies of this phenomenon, which led to the recognition that it is related to the induction of large, abnormal ionic currents by the structurally altered PrP molecules. Our results suggest a model in which the flexible N-terminal domain of PrPC serves as a toxic effector which is regulated by intramolecular interactions with the globular C-terminal domain. Taken together, these two areas of study have provided important clues to underlying cellular and molecular mechanisms of prion neurotoxicity. Nevertheless, much remains to be done on this next frontier of prion science.
Keywords: antibody; cell culture; dendrite; glutamate; ionic current; mitogen-activated protein kinase (MAPK); neurodegeneration; neurotoxicity; prion; spine; synapse; transgenic mouse.
© 2018 International Society of Neuropathology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Prions activate a p38 MAPK synaptotoxic signaling pathway.PLoS Pathog. 2018 Sep 20;14(9):e1007283. doi: 10.1371/journal.ppat.1007283. eCollection 2018 Sep. PLoS Pathog. 2018. PMID: 30235355 Free PMC article.
-
Recent advances in the histo-molecular pathology of human prion disease.Brain Pathol. 2019 Mar;29(2):278-300. doi: 10.1111/bpa.12695. Epub 2019 Jan 22. Brain Pathol. 2019. PMID: 30588685 Free PMC article. Review.
-
An inter-domain regulatory mechanism controls toxic activities of PrPC.Prion. 2017 Nov 2;11(6):388-397. doi: 10.1080/19336896.2017.1384894. Epub 2017 Nov 2. Prion. 2017. PMID: 28960140 Free PMC article.
-
Pathogenic prions deviate PrP(C) signaling in neuronal cells and impair A-beta clearance.Cell Death Dis. 2013 Jan 10;4(1):e456. doi: 10.1038/cddis.2012.195. Cell Death Dis. 2013. PMID: 23303130 Free PMC article.
-
The prion gene complex encoding PrP(C) and Doppel: insights from mutational analysis.Gene. 2001 Sep 5;275(1):1-18. doi: 10.1016/s0378-1119(01)00627-8. Gene. 2001. PMID: 11574147 Review.
Cited by
-
Human prion diseases and the prion protein - what is the current state of knowledge?Transl Neurosci. 2023 Oct 16;14(1):20220315. doi: 10.1515/tnsci-2022-0315. eCollection 2023 Jan 1. Transl Neurosci. 2023. PMID: 37854584 Free PMC article. Review.
-
Potential Therapeutic Use of Stem Cells for Prion Diseases.Cells. 2023 Oct 7;12(19):2413. doi: 10.3390/cells12192413. Cells. 2023. PMID: 37830627 Free PMC article. Review.
-
Recent advances on the molecular pathogenesis of prion diseases.Brain Pathol. 2019 Mar;29(2):245-247. doi: 10.1111/bpa.12693. Brain Pathol. 2019. PMID: 30588674 Free PMC article. No abstract available.
-
Transgenic Overexpression of the Disordered Prion Protein N1 Fragment in Mice Does Not Protect Against Neurodegenerative Diseases Due to Impaired ER Translocation.Mol Neurobiol. 2020 Jun;57(6):2812-2829. doi: 10.1007/s12035-020-01917-2. Epub 2020 May 4. Mol Neurobiol. 2020. PMID: 32367491 Free PMC article.
-
A Systematic Review of Sporadic Creutzfeldt-Jakob Disease: Pathogenesis, Diagnosis, and Therapeutic Attempts.Neurol Int. 2024 Sep 20;16(5):1039-1065. doi: 10.3390/neurolint16050079. Neurol Int. 2024. PMID: 39311352 Free PMC article. Review.
References
-
- Alam J, Blackburn K, Patrick D (2017) Neflamapimod: clinical phase 2b‐ready oral small molecule inhibitor of p38α to reverse synaptic dysfunction in early Alzheimer's disease. J Prev Alzheimers Dis 4:273–278. - PubMed
-
- Ambrosi G, Cerri S, Blandini F (2014) A further update on the role of excitotoxicity in the pathogenesis of Parkinson's disease. J Neural Transm (Vienna) 121:849–859. - PubMed
-
- Belichenko PV, Brown D, Jeffrey M, Fraser JR (2000) Dendritic and synaptic alterations of hippocampal pyramidal neurones in scrapie‐infected mice. Neuropathol Appl Neurobiol 26:143–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

