The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications
- PMID: 30589505
- PMCID: PMC6198240
- DOI: 10.1002/cam4.1501
The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications
Abstract
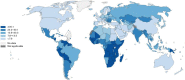
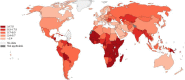
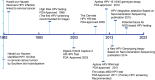
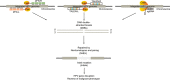
Cervical cancer is the third most common cancer in women worldwide, with concepts and knowledge about its prevention and treatment evolving rapidly. Human papillomavirus (HPV) has been identified as a major factor that leads to cervical cancer, although HPV infection alone cannot cause the disease. In fact, HPV-driven cancer is a small probability event because most infections are transient and could be cleared spontaneously by host immune system. With persistent HPV infection, decades are required for progression to cervical cancer. Therefore, this long time window provides golden opportunity for clinical intervention, and the fundament here is to elucidate the carcinogenic pattern and applicable targets during HPV-host interaction. In this review, we discuss the key factors that contribute to the persistence of HPV and cervical carcinogenesis, emerging new concepts and technologies for cancer interventions, and more urgently, how these concepts and technologies might lead to clinical precision medicine which could provide prediction, prevention, and early treatment for patients.
Keywords: Cervical carcinogenesis; HPV integration; NGS‐based HPV testing; cervical screening; genome editing tools.
© 2018 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Jemal, A. , Bray F., Center M. M., Ferlay J., Ward E., and Forman D.. 2011. Global cancer statistics. CA Cancer J. Clin. 61:69–90. - PubMed
-
- Crosbie, E. J. , Einstein M. H., Franceschi S., and Kitchener H. C.. 2013. Human papillomavirus and cervical cancer. Lancet 382(9895):889–99. - PubMed
-
- Siegel, R. L. , Miller K. D., and Jemal A.. 2016. Cancer statistics, 2016. CA Cancer J. Clin. 66:7–30. - PubMed

