Development of a High-Throughput Lysyl Hydroxylase (LH) Assay and Identification of Small-Molecule Inhibitors against LH2
- PMID: 30589612
- PMCID: PMC6535306
- DOI: 10.1177/2472555218817057
Development of a High-Throughput Lysyl Hydroxylase (LH) Assay and Identification of Small-Molecule Inhibitors against LH2
Abstract
Lysyl hydroxylase-2 (LH2) catalyzes the hydroxylation of telopeptidyl lysine residues on collagen, leading to the formation of stable collagen cross-links that connect collagen molecules and stabilize the extracellular matrix. High levels of LH2 have been reported in the formation and stabilization of hydroxylysine aldehyde-derived collagen cross-links (HLCCs), leading to fibrosis and cancer metastasis in certain tissues. Identification of small-molecule inhibitors targeting LH2 activity requires a robust and suitable assay system, which is currently lacking. Thus, despite being a promising target for these diseases, small-molecule inhibitors for LH2 have yet to be reported. Therefore, we developed a luminescence-based strategy to monitor LH activity and validated its ability to identify new inhibitors in a screen of approximately 65,000 compounds against LH2. Primary hits were confirmed using the same LH assay against mimiviral L230. This newly developed LH assay is robust, suitable for high-throughput screening, and able to identify potent specific inhibitors of LH2.
Keywords: cancer; high-throughput screen; luminescence; lysyl hydroxylase-2 (LH2); succinate detection.
Conflict of interest statement
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
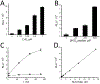


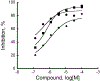
References
-
- Loenarz C; Schofield CJ Expanding Chemical Biology of 2-Oxoglutarate Oxygenases. Nat. Chem. Biol 2008, 4 (3), 152–6. - PubMed
-
- Takaluoma K; Lantto J; Myllyharju J Lysyl Hydroxylase 2 Is a Specific Telopeptide Hydroxylase, While All Three Isoenzymes Hydroxylate Collagenous Sequences. Matrix Biol. 2007, 26 (5), 396–403. - PubMed
-
- Van der Slot AJ; Zuurmond AM; van den Bogaerdt AJ; et al. Increased Formation of Pyridinoline Cross-Links Due to Higher Telopeptide Lysyl Hydroxylase Levels Is a General Fibrotic Phenomenon. Matrix Biol. 2004, 23 (4), 251–7. - PubMed
-
- Van der Slot AJ; Zuurmond AM; Bardoel AF; et al. Identification of Plod2 as Telopeptide Lysyl Hydroxylase, an Important Enzyme in Fibrosis. J. Biol. Chem 2003, 278 (42), 40967–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

