Toward the Bionic Face: A Novel Neuroprosthetic Device Paradigm for Facial Reanimation Consisting of Neural Blockade and Functional Electrical Stimulation
- PMID: 30589784
- PMCID: PMC6311722
- DOI: 10.1097/PRS.0000000000005164
Toward the Bionic Face: A Novel Neuroprosthetic Device Paradigm for Facial Reanimation Consisting of Neural Blockade and Functional Electrical Stimulation
Abstract
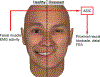
Background: Facial palsy is a devastating condition potentially amenable to rehabilitation by functional electrical stimulation. Herein, a novel paradigm for unilateral facial reanimation using an implantable neuroprosthetic device is proposed and its feasibility demonstrated in a live rodent model. The paradigm comprises use of healthy-side electromyographic activity as control inputs to a system whose outputs are neural stimuli to effect symmetric facial displacements. The vexing issue of suppressing undesirable activity resulting from aberrant neural regeneration (synkinesis) or nerve transfer procedures is addressed using proximal neural blockade.
Methods: Epimysial and nerve cuff electrode arrays were implanted in the faces of Wistar rats. Stimuli were delivered to evoke blinks and whisks of various durations and amplitudes. The dynamic relation between electromyographic signals and facial displacements was modeled, and model predictions were compared against measured displacements. Optimal parameters to achieve facial nerve blockade by means of high-frequency alternating current were determined, and the safety of continuous delivery was assessed.
Results: Electrode implantation was well tolerated. Blinks and whisks of tunable amplitudes and durations were evoked by controlled variation of neural stimuli parameters. Facial displacements predicted from electromyographic input modelling matched those observed with a variance-accounted-for exceeding 96 percent. Effective and reversible facial nerve blockade in awake behaving animals was achieved, without detrimental effect noted from long-term continual use.
Conclusions: Proof-of-principle of rehabilitation of hemifacial palsy by means of a neuroprosthetic device has been demonstrated. The use of proximal neural blockade coupled with distal functional electrical stimulation may have relevance to rehabilitation of other peripheral motor nerve deficits.
Figures


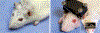










Similar articles
-
Brief electrical stimulation and synkinesis after facial nerve crush injury: a randomized prospective animal study.J Otolaryngol Head Neck Surg. 2018 Mar 7;47(1):20. doi: 10.1186/s40463-018-0264-0. J Otolaryngol Head Neck Surg. 2018. PMID: 29514718 Free PMC article.
-
Potential of an electric prosthesis for dynamic facial reanimation.Otolaryngol Head Neck Surg. 2011 Sep;145(3):365-8. doi: 10.1177/0194599811406065. Epub 2011 Jun 2. Otolaryngol Head Neck Surg. 2011. PMID: 21636836 Review.
-
Feasibility of bionic reanimation of a paralyzed face: a preliminary study of functional electrical stimulation of a paralyzed facial muscle controlled with the electromyography of the contralateral healthy hemiface.Plast Reconstr Surg. 2010 Aug;126(2):81e-83e. doi: 10.1097/PRS.0b013e3181df6ff3. Plast Reconstr Surg. 2010. PMID: 20679796 No abstract available.
-
Recovery of facial expressions using functional electrical stimulation after full-face transplantation.J Neuroeng Rehabil. 2018 Mar 6;15(1):15. doi: 10.1186/s12984-018-0356-0. J Neuroeng Rehabil. 2018. PMID: 29510722 Free PMC article.
-
Facial reanimation: an invited review and commentary.Arch Facial Plast Surg. 2008 Nov-Dec;10(6):413-7. doi: 10.1001/archfaci.10.6.413. Arch Facial Plast Surg. 2008. PMID: 19018064 Review. No abstract available.
Cited by
-
Reduction of the onset response in kilohertz frequency alternating current nerve block with amplitude ramps from non-zero amplitudes.J Neuroeng Rehabil. 2019 Jun 28;16(1):80. doi: 10.1186/s12984-019-0554-4. J Neuroeng Rehabil. 2019. PMID: 31253152 Free PMC article.
-
Towards the clinical translation of optogenetic skeletal muscle stimulation.Pflugers Arch. 2020 May;472(5):527-545. doi: 10.1007/s00424-020-02387-0. Epub 2020 May 15. Pflugers Arch. 2020. PMID: 32415463 Free PMC article. Review.
-
Continual rehabilitation motivation of patients with postparalytic facial nerve syndrome.Eur Arch Otorhinolaryngol. 2022 Jan;279(1):481-491. doi: 10.1007/s00405-021-06895-2. Epub 2021 May 24. Eur Arch Otorhinolaryngol. 2022. PMID: 34027598 Free PMC article.
-
Human eyelid behavior is driven by segmental neural control of the orbicularis oculi.Proc Natl Acad Sci U S A. 2025 Aug 12;122(32):e2508058122. doi: 10.1073/pnas.2508058122. Epub 2025 Aug 7. Proc Natl Acad Sci U S A. 2025. PMID: 40773233 Free PMC article.
-
Machine-Learning-Based Detecting of Eyelid Closure and Smiling Using Surface Electromyography of Auricular Muscles in Patients with Postparalytic Facial Synkinesis: A Feasibility Study.Diagnostics (Basel). 2023 Feb 2;13(3):554. doi: 10.3390/diagnostics13030554. Diagnostics (Basel). 2023. PMID: 36766657 Free PMC article.
References
-
- Coulson SE, O’Dwyer N,J, Adams RD, Croxson GR Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol 2004;25:1014–1019. - PubMed
-
- Ryzenman JM, Pensak ML, Tew JM Jr. Facial paralysis and surgical rehabilitation: a quality of life analysis in a cohort of 1,595 patients after acoustic neuroma surgery. Otol Neurotol 2005;26:516–521; discussion 521. - PubMed
-
- Lassaletta L, Alfonso C, Del Rio, L., Roda JM, Gavilan J Impact of facial dysfunction on quality of life after vestibular schwannoma surgery. Annals of Otology, Rhinology & Laryngology 2006;115:694–698. - PubMed
-
- Mehta RP, WernickRobinson M, Hadlock TA Validation of the Synkinesis Assessment Questionnaire. Laryngoscope 2007;117:923–926. - PubMed
-
- Hadlock T, Cheney ML Facial reanimation: an invited review and commentary. Arch Facial Plast Surg 2008;10:413–417. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

