Conformational fingerprint of blood and tissue ACEs: Personalized approach
- PMID: 30589901
- PMCID: PMC6307727
- DOI: 10.1371/journal.pone.0209861
Conformational fingerprint of blood and tissue ACEs: Personalized approach
Abstract
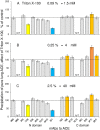
Background: The pattern of binding of monoclonal antibodies (mAbs) to 18 epitopes on human angiotensin I-converting enzyme (ACE)-"conformational fingerprint of ACE"-is a sensitive marker of subtle conformational changes of ACE due to mutations, different glycosylation in various cells, the presence of ACE inhibitors and specific effectors, etc.
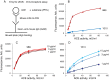
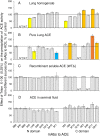
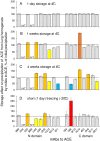
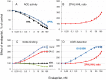
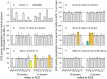
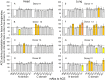
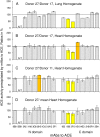
Methodology/principal findings: We described in detail the methodology of the conformational fingerprinting of human blood and tissue ACEs that allows detecting differences in surface topography of ACE from different tissues, as well detecting inter-individual differences. Besides, we compared the sensitivity of the detection of ACE inhibitors in the patient's plasma using conformational fingerprinting of ACE (with only 2 mAbs to ACE, 1G12 and 9B9) and already accepted kinetic assay and demonstrated that the mAbs-based assay is an order of magnitude more sensitive. This approach is also very effective in detection of known (like bilirubin and lysozyme) and still unknown ACE effectors/inhibitors which nature and set could vary in different tissues or different patients.
Conclusions/significance: Phenotyping of ACE (and conformational fingerprinting of ACE as a part of this novel approach for characterization of ACE) in individuals really became informative and clinically relevant. Appreciation (and counting on) of inter-individual differences in ACE conformation and accompanying effectors make the application of this approach for future personalized medicine with ACE inhibitors more accurate. This (or similar) methodology can be applied to any enzyme/protein for which there is a number of mAbs to its different epitopes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Sturrock ED, Anthony CS, Danilov SM (2012) Peptidyl-dipeptidase A/Angiotensin I-converting enzyme In: Rawlings ND; Salvesen G, ed. Handbook of Proteolytic Enzymes, 3rd edn Oxford, Academic Press: 480–494.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

