Month 12 Outcomes After Treatment Change at Month 6 Among Poor Responders to Aflibercept or Bevacizumab in Eyes With Macular Edema Secondary to Central or Hemiretinal Vein Occlusion: A Secondary Analysis of the SCORE2 Study
- PMID: 30589922
- PMCID: PMC6439712
- DOI: 10.1001/jamaophthalmol.2018.6111
Month 12 Outcomes After Treatment Change at Month 6 Among Poor Responders to Aflibercept or Bevacizumab in Eyes With Macular Edema Secondary to Central or Hemiretinal Vein Occlusion: A Secondary Analysis of the SCORE2 Study
Abstract
Importance: Information is needed to assess switching treatment in eyes with a poor response to 6 months of monthly administration of aflibercept or bevacizumab for macular edema from central retinal vein occlusion (CRVO) or hemiretinal vein occlusion (HRVO).
Objective: To investigate visual acuity letter score (VALS) and central subfield thickness (CST) changes from month 6 to 12 among eyes with a poor response at month 6 to monthly dosing of aflibercept or bevacizumab in the Study of Comparative Treatments for Retinal Vein Occlusion 2.
Design, setting, and participants: This secondary analysis of the Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) was conducted at 66 private practice or academic centers in the United States. Participants included 49 patients (1 eye from each patient evaluated) with CRVO- or HRVO-associated macular edema and a protocol-defined poor response to aflibercept or bevacizumab treatment at month 6. The first month 6 visit occurred on September 8, 2015, and the last month 12 visit occurred on October 24, 2016.
Interventions: Treatment in eyes receiving monthly aflibercept was switched to a dexamethasone implant at month 6 and, if needed, at months 9, 10, or 11. Treatment in eyes receiving monthly bevacizumab was switched to aflibercept at months 6, 7, and 8, and then to a treat-and-extend aflibercept regimen until month 12.
Main outcomes and measures: Change from month 6 to 12 in VALS and CST.
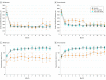
Results: Of the 49 participants at month 6, aflibercept treatment had failed in 14 (6 [43%] women; mean [SD] age, 70.4 [13.0] years). Bevacizumab treatment had failed in 35 patients (16 [46%] women; mean age, 70.0 [13.2] years). In 14 eyes with treatment switched from aflibercept to dexamethasone, the estimated mean change from month 6 to 12 in VALS was 2.63 (95% CI, -3.29 to 8.56; P = .37) and 46.0 μm (95% CI, -80.9 to 172.9 μm; P = .46) for CST. In 35 eyes with treatment switched from bevacizumab to aflibercept, the estimated mean change from month 6 to 12 in VALS was 10.27 (95% CI, 6.05-14.49; P < .001) and -125.4 μm (95% CI, -180.9 to -69.9 μm; P < .001) for CST.
Conclusions and relevance: Eyes treated with aflibercept after a poor response to bevacizumab had improvement in VALS and CST. Few eyes had a poor response to aflibercept, and therefore, few eyes were switched to dexamethasone. Caution is warranted in interpreting these results owing to the small number of eyes and lack of comparison groups. These factors preclude definitive assessment of whether the switching strategy is superior to maintaining treatment.
Conflict of interest statement
Figures
References
-
- Scott IU, VanVeldhuisen PC, Ip MS, et al. ; SCORE2 Investigator Group . Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA. 2017;317(20):2072-2087. doi: 10.1001/jama.2017.4568 - DOI - PMC - PubMed
-
- Scott IU, VanVeldhuisen PC, Ip MS, et al. ; SCORE2 Investigator Group . Comparison of monthly vs treat-and-extend regimens for individuals with macular edema who respond well to anti-vascular endothelial growth factor medications: secondary outcomes from the SCORE2 randomized clinical trial. JAMA Ophthalmol. 2018;136(4):337-345. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

