Neuroimmune signaling in alcohol use disorder
- PMID: 30590091
- PMCID: PMC6946054
- DOI: 10.1016/j.pbb.2018.12.007
Neuroimmune signaling in alcohol use disorder
Abstract
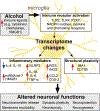
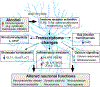
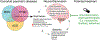
Alcohol use disorder (AUD) is a widespread disease with limited treatment options. Targeting the neuroimmune system is a new avenue for developing or repurposing effective pharmacotherapies. Alcohol modulates innate immune signaling in different cell types in the brain by altering gene expression and the molecular pathways that regulate neuroinflammation. Chronic alcohol abuse may cause an imbalance in neuroimmune function, resulting in prolonged perturbations in brain function. Likewise, manipulating the neuroimmune system may change alcohol-related behaviors. Psychiatric disorders that are comorbid with AUD, such as post-traumatic stress disorder, major depressive disorder, and other substance use disorders, may also have underlying neuroimmune mechanisms; current evidence suggests that convergent immune pathways may be involved in AUD and in these comorbid disorders. In this review, we provide an overview of major neuroimmune cell-types and pathways involved in mediating alcohol behaviors, discuss potential mechanisms of alcohol-induced neuroimmune activation, and present recent clinical evidence for candidate immune-related drugs to treat AUD.
Keywords: Addiction; Alcohol; Astrocyte; Comorbidity; Microglia; Neuroimmune.
Copyright © 2018. Published by Elsevier Inc.
Figures
References
-
- Aal-Aaboda M, Alhaddad H, Osowik F, Nauli SM, Sari Y, 2015. Effects of (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. J. Neurosci. Res. 93, 930–937. 10.1002/jnr.23554. - DOI - PMC - PubMed
-
- Abbasi S-H, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S, 2012. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J. Affect. Disord. 141, 308–314. 10.1016/j.jad.2012.03.033. - DOI - PubMed
-
- Adedayo AD, Aderinola AA, Adekilekun TA, Olaolu OO, Olanike AM, Olayemi IK, 2018. Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats. Anat. Cell Biol. 51, 41–51. 10.5115/acb.2018.51.1.41. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

