Regulation of endothelial barrier integrity by redox-dependent nitric oxide signaling: Implication in traumatic and inflammatory brain injuries
- PMID: 30590116
- PMCID: PMC6546177
- DOI: 10.1016/j.niox.2018.12.007
Regulation of endothelial barrier integrity by redox-dependent nitric oxide signaling: Implication in traumatic and inflammatory brain injuries
Abstract
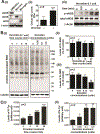
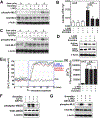
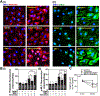
Nitric oxide (NO) synthesized by eNOS plays a key role in regulation of endothelial barrier integrity but underlying cell signaling pathway is not fully understood at present. Here, we report opposing roles of two different redox-dependent NO metabolites; peroxynitrite (ONOO-) vs. S-nitrosoglutathione (GSNO), in cell signaling pathways for endothelial barrier disruption. In cultured human brain microvessel endothelial cells (hBMVECs), thrombin induced F-actin stress fiber formation causes barrier disruption via activating eNOS. Thrombin induced eNOS activity participated in cell signaling (e.g. RhoA and calcium influx mediated phosphorylation of myosin light chain) for F-actin stress fiber formation by increasing ONOO- levels. On the other hand, thrombin had no effect on intracellular levels of S-nitrosoglutathione (GSNO), another cellular NO metabolite. However, exogenous GSNO treatment attenuated the thrombin-induced cell signaling pathways for endothelial barrier disruption, thus suggesting the role of a shift of NO metabolism (GSNO vs. ONOO-) toward ONOO- synthesis in cell signaling for endothelial barrier disruption. Consistent with these in vitro studies, in animal models of traumatic brain injury and experimental autoimmune encephalomyelitis (EAE), ONOO- scavenger treatment as well as GSNO treatment were effective for attenuation of BBB leakage, edema formation, and CNS infiltration of mononuclear cells. Taken together, these data document that eNOS-mediated NO production and following redox-dependent NO metabolites (ONOO- vs. GSNO) are potential therapeutic target for CNS microvascular disease (traumatic and inflammatory) pathologies.
Keywords: Actin stress fiber; Brain endothelial barrier; Endothelial nitric oxide synthase (eNOS); Nitric oxide; Peroxynitrite (ONOOˉ); S-nitrosoglutathione (GSNO).
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

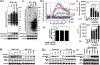

Similar articles
-
S-Nitrosoglutathione Mimics the Beneficial Activity of Endothelial Nitric Oxide Synthase-Derived Nitric Oxide in a Mouse Model of Stroke.J Stroke Cerebrovasc Dis. 2019 Dec;28(12):104470. doi: 10.1016/j.jstrokecerebrovasdis.2019.104470. Epub 2019 Nov 1. J Stroke Cerebrovasc Dis. 2019. PMID: 31680031
-
S-nitrosoglutathione reduces oxidative injury and promotes mechanisms of neurorepair following traumatic brain injury in rats.J Neuroinflammation. 2011 Jul 6;8:78. doi: 10.1186/1742-2094-8-78. J Neuroinflammation. 2011. PMID: 21733162 Free PMC article.
-
The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke.J Neurochem. 2012 Nov;123 Suppl 2(Suppl 2):86-97. doi: 10.1111/j.1471-4159.2012.07947.x. J Neurochem. 2012. PMID: 23050646 Free PMC article.
-
Neuroprotective properties of nitric oxide.Ann N Y Acad Sci. 1999;890:301-11. doi: 10.1111/j.1749-6632.1999.tb08007.x. Ann N Y Acad Sci. 1999. PMID: 10668435 Review.
-
The redox pathway of S-nitrosoglutathione, glutathione and nitric oxide in cell to neuron communications.Free Radic Res. 1999 Dec;31(6):641-50. doi: 10.1080/10715769900301211. Free Radic Res. 1999. PMID: 10630687 Review.
Cited by
-
Molecular Dambusters: What Is Behind Hyperpermeability in Bradykinin-Mediated Angioedema?Clin Rev Allergy Immunol. 2021 Jun;60(3):318-347. doi: 10.1007/s12016-021-08851-8. Epub 2021 Mar 16. Clin Rev Allergy Immunol. 2021. PMID: 33725263 Free PMC article. Review.
-
Angiotensin II-induced endothelial dysfunction: Impact of sex, genetic background, and rho kinase.Physiol Rep. 2022 Jun;10(11):e15336. doi: 10.14814/phy2.15336. Physiol Rep. 2022. PMID: 35681278 Free PMC article.
-
Hydroxyethylstarch revisited for acute brain injury treatment.Neural Regen Res. 2021 Jul;16(7):1372-1376. doi: 10.4103/1673-5374.300978. Neural Regen Res. 2021. PMID: 33318420 Free PMC article. Review.
-
Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress.J Biol Chem. 2021 Jan-Jun;296:100171. doi: 10.1074/jbc.RA120.015059. Epub 2020 Dec 14. J Biol Chem. 2021. PMID: 33298523 Free PMC article.
-
Salivary-Gland-Mediated Nitrate Recirculation as a Modulator for Cardiovascular Diseases.Biomolecules. 2025 Mar 19;15(3):439. doi: 10.3390/biom15030439. Biomolecules. 2025. PMID: 40149975 Free PMC article. Review.
References
-
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O’Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR, Engaging neuroscience to advance translational research in brain barrier biology, Nat Rev Neurosci 12 (2011) 169–82. - PMC - PubMed
-
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME, Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation, Stroke 31 (2000) 1965–72; discussion 1972–3. - PubMed
-
- Olah L, Wecker S, Hoehn M, Secondary deterioration of apparent diffusion coefficient after 1-hour transient focal cerebral ischemia in rats, J Cereb Blood Flow Metab 20 (2000) 1474–82. - PubMed
-
- Kaur J, Tuor UI, Zhao Z, Petersen J, Jin AY, Barber PA, Quantified T1 as an adjunct to apparent diffusion coefficient for early infarct detection: a high-field magnetic resonance study in a rat stroke model, Int J Stroke 4 (2009) 159–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

