Insights into photoreceptor ciliogenesis revealed by animal models
- PMID: 30590118
- PMCID: PMC6707082
- DOI: 10.1016/j.preteyeres.2018.12.004
Insights into photoreceptor ciliogenesis revealed by animal models
Abstract
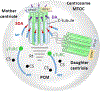
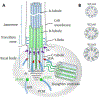
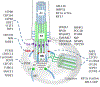
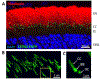
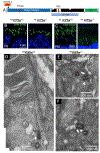
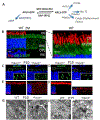
Photoreceptors are polarized neurons, with very specific subcellular compartmentalization and unique requirements for protein expression and trafficking. Each photoreceptor contains an outer segment, the site of photon capture that initiates vision, an inner segment that houses the biosynthetic machinery and a synaptic terminal for signal transmission to downstream neurons. Outer segments and inner segments are connected by a connecting cilium (CC), the equivalent of a transition zone (TZ) of primary cilia. The connecting cilium is part of the basal body/axoneme backbone that stabilizes the outer segment. This report will update the reader on late developments in photoreceptor ciliogenesis and transition zone formation, specifically in mouse photoreceptors, focusing on early events in photoreceptor ciliogenesis. The connecting cilium, an elongated and narrow structure through which all outer segment proteins and membrane components must traffic, functions as a gate that controls access to the outer segment. Here we will review genes and their protein products essential for basal body maturation and for CC/TZ genesis, sorted by phenotype. Emphasis is given to naturally occurring mouse mutants and gene knockouts that interfere with CC/TZ formation and ciliogenesis.
Keywords: Centrosome; Distal and subdistal appendages; Knockout mouse models; Microtubules and microtubule organization center; Mother and daughter centrioles; Pericentriolar matrix; Photoreceptors; Transition zone.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures











References
-
- Acland GM, Blanton SH, Hershfield B, and Aguiree GD (1994). XLPRA: a canine retinal degeneration inherited as an X-linked trait. Am. J. Med. Genet 52, 27–33. - PubMed
-
- Adly N, Alhashem A, Ammari A, and Alkuraya FS (2014). Ciliary genes TBC1D32/C6orf170 and SCLT1 are mutated in patients with OFD type IX. Hum. Mutat 35, 36–40. - PubMed
-
- Airik R, Slaats GG, Guo Z, Weiss AC, Khan N, Ghosh A, Hurd TW, Bekker-Jensen S, Schroder JM, Elledge SJ, Andersen JS, Kispert A, Castelli M, Boletta A, Giles RH, and Hildebrandt F (2014). Renal-retinal ciliopathy gene Sdccag8 regulates DNA damage response signaling. J. Am. Soc. Nephrol 25, 2573–2583. - PMC - PubMed
-
- Aldaz H, Rice LM, Stearns T, and Agard DA (2005). Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature 435, 523–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

