Switching From a Protease Inhibitor-based Regimen to a Dolutegravir-based Regimen: A Randomized Clinical Trial to Determine the Effect on Peripheral Blood and Ileum Biopsies From Antiretroviral Therapy-suppressed Human Immunodeficiency Virus-infected Individuals
- PMID: 30590412
- PMCID: PMC6763634
- DOI: 10.1093/cid/ciy1095
Switching From a Protease Inhibitor-based Regimen to a Dolutegravir-based Regimen: A Randomized Clinical Trial to Determine the Effect on Peripheral Blood and Ileum Biopsies From Antiretroviral Therapy-suppressed Human Immunodeficiency Virus-infected Individuals
Abstract
Background: Optimization of combination antiretroviral therapy (cART) can impact the human immunodeficiency virus (HIV) reservoir. We evaluated the effect on the HIV reservoir in peripheral blood and ileum biopsies in patients switching from boosted protease inhibitor (PI/r)-based therapy to dolutegravir (DTG)-based therapy.
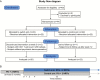
Methods: Impact of Integrase-inhibitor DOlutegravir On the viral Reservoir (INDOOR) is a phase 4 open-label clinical trial that randomly included 42 HIV type 1-infected individuals on effective cART: 20 who switched from PI/r-based to DTG-based cART (switch group), and 22 who remained in PI/r-based regimens (control group). We analyzed blood and ileum biopsies to quantify episomal, total, and integrated HIV DNA, cell-associated HIV RNA, residual plasma viremia, T-cell subsets, cell activation, and inflammation markers.
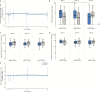
Results: There were no related adverse events or treatment discontinuations due to drug intolerance. The HIV reservoir was consistently larger in ileal than in peripheral CD4+ T cells in both groups (P < .01). Residual viremia in plasma decreased in the switch group (P = .03). However, we did not observe significant longitudinal changes in low-level viral replication, total and integrated HIV reservoir, HIV transcription, T-cell maturation subsets, immunoactivation markers, inflammatory soluble proteins, or cellular markers of latently infected cells.
Conclusions: The INDOOR study is the first evaluation of changes in HIV reservoir size in ileum biopsies and in peripheral blood in individuals switched from PI/r- to DTG-based cART. Although this switch was safe and well tolerated, it had no impact on a large array of immunological and inflammatory markers or on HIV reservoir markers in peripheral or in ileal CD4+ T cells.
Clinical trials registration: EudraCT 2014-004331-39.
Keywords: HIV reservoir; dolutegravir; ileum biopsies; immunological and inflammation markers; switching.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- Johnson VA. Combination therapy: more effective control of HIV type 1? AIDS Res Hum Retroviruses 1994; 10:907–12. - PubMed
-
- Palella FJ Jr, Armon C, Buchacz K, et al. . HOPS Investigators Factors associated with mortality among persistently viraemic triple-antiretroviral-class-experienced patients receiving antiretroviral therapy in the HIV Outpatient Study (HOPS). J Antimicrob Chemother 2014; 69:2826–34. - PubMed
-
- Lang S, Mary-Krause M, Cotte L, et al. . Clinical Epidemiology Group of the French Hospital Database on HIV Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med 2010; 170:1228–38. - PubMed
-
- Potard V, Simon A, Lacombe J-M, Parienti J-J, Costagliola D; French Hospital Database on HIV (FHDH-ANRS CO4) Switching to raltegravir from a virologically effective boosted protease inhibitor regimen: a comparative effectiveness analysis from the French Hospital Database on HIV (FHDH-ANRS CO4). Clin Infect Dis 2016; 63:1254–61. - PubMed
-
- Negredo E, Estrada V, Domingo P, et al. . Switching from a ritonavir-boosted PI to dolutegravir as an alternative strategy in virologically suppressed HIV-infected individuals. J Antimicrob Chemother 2016; 72:844–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

