Kctd13-deficient mice display short-term memory impairment and sex-dependent genetic interactions
- PMID: 30590535
- PMCID: PMC6489413
- DOI: 10.1093/hmg/ddy436
Kctd13-deficient mice display short-term memory impairment and sex-dependent genetic interactions
Abstract
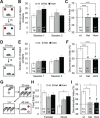
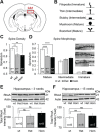
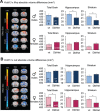
The 16p11.2 BP4-BP5 deletion and duplication syndromes are associated with a complex spectrum of neurodevelopmental phenotypes that includes developmental delay and autism spectrum disorder, with a reciprocal effect on head circumference, brain structure and body mass index. Mouse models of the 16p11.2 copy number variant have recapitulated some of the patient phenotypes, while studies in flies and zebrafish have uncovered several candidate contributory genes within the region, as well as complex genetic interactions. We evaluated one of these loci, KCTD13, by modeling haploinsufficiency and complete knockout in mice. In contrast to the zebrafish model, and in agreement with recent data, we found normal brain structure in heterozygous and homozygous mutants. However, recapitulating previously observed genetic interactions, we discovered sex-specific brain volumetric alterations in double heterozygous Kctd13xMvp and Kctd13xLat mice. Behavioral testing revealed a significant deficit in novel object recognition, novel location recognition and social transmission of food preference in Kctd13 mutants. These phenotypes were concomitant with a reduction in density of mature spines in the hippocampus, but potentially independent of RhoA abundance, which was unperturbed postnatally in our mutants. Furthermore, transcriptome analyses from cortex and hippocampus highlighted the dysregulation of pathways important in neurodevelopment, the most significant of which was synaptic formation. Together, these data suggest that KCTD13 contributes to the neurocognitive aspects of patients with the BP4-BP5 deletion, likely through genetic interactions with other loci.
© The Author(s) 2018. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Grayton H.M., Fernandes C., Rujescu D. and Collier D.A. (2012) Copy number variations in neurodevelopmental disorders. Prog. Neurobiol., 99, 81–91. - PubMed
-
- Weiss L.A., Shen Y.P., Korn J.M., Arking D.E., Miller D.T., Fossdal R., Saemundsen E., Stefansson H., Ferreira M.A.R., Green T. et al. (2008) Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med., 358, 667–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

