Characterization of Human Adrenal Steroidogenesis During Fetal Development
- PMID: 30590593
- PMCID: PMC6456011
- DOI: 10.1210/jc.2018-01759
Characterization of Human Adrenal Steroidogenesis During Fetal Development
Abstract
Context: The endocrine function of human fetal adrenals (HFAs) is activated already during first trimester, but adrenal steroidogenesis during fetal life is not well characterized.
Objective: This study aimed to investigate HFA steroidogenesis by analyzing adrenal glands from first and second trimesters.
Design and setting: Male and female HFA from gestational weeks (GWs) 8 to 19 were examined, including a total of 101 samples from 83 fetuses.
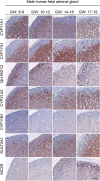
Main outcome measure(s): Expression level of steroidogenic genes and protein expression/localization were determined by quantitative PCR and immunohistochemistry, respectively, and intra-adrenal steroid levels were quantified by LC-MS/MS.
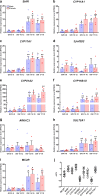
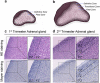
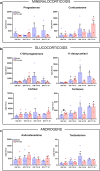
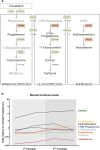
Results: Transcriptional levels of StAR, CYP11A1, CYP17A1, CYP21A2, CYP11B1/2, and SULT2A1 were significantly higher in second trimester compared to first trimester (P < 0.05), whereas expression levels of 3β-HSD2 and ARK1C3 were unaltered between GWs 8 and 19. All investigated steroidogenic proteins were expressed in a distinct pattern throughout the investigated period, with most enzymes expressed primarily in the fetal zone, except 3β-HSD1/2, which was expressed mainly in the definitive zone. Abundant steroidogenic enzyme expression was reflected in overall high intra-adrenal tissue concentrations of mineralocorticoids, glucocorticoids, and androgens; cortisol was the most abundant (1071 to 2723 ng/g tissue), and testosterone levels were the lowest (2 to 14 ng/g tissue).
Conclusions: The expression profiles of HFA steroidogenic enzymes are distinct from first to second trimester, with no major differences between male and female samples. Intra-adrenal steroid hormone concentrations confirm that cortisol is produced throughout first and second trimesters, suggesting continued regulation of the hypothalamus-pituitary-adrenal axis during this entire period.
Copyright © 2019 Endocrine Society.
Figures
Similar articles
-
The human fetal adrenal produces cortisol but no detectable aldosterone throughout the second trimester.BMC Med. 2018 Feb 12;16(1):23. doi: 10.1186/s12916-018-1009-7. BMC Med. 2018. PMID: 29429410 Free PMC article.
-
Expression of steroidogenic enzymes in human placenta according to the gestational age.Mol Med Rep. 2019 May;19(5):3903-3911. doi: 10.3892/mmr.2019.10048. Epub 2019 Mar 15. Mol Med Rep. 2019. PMID: 30896833
-
Equine fetal adrenal, gonadal and placental steroidogenesis.Reproduction. 2017 Oct;154(4):445-454. doi: 10.1530/REP-17-0239. Reproduction. 2017. PMID: 28878092
-
Lipoprotein utilization and cholesterol synthesis by the human fetal adrenal gland.Endocr Rev. 1981 Summer;2(3):306-26. doi: 10.1210/edrv-2-3-306. Endocr Rev. 1981. PMID: 6268397 Review.
-
Steroidogenesis in the skin: implications for local immune functions.J Steroid Biochem Mol Biol. 2013 Sep;137:107-23. doi: 10.1016/j.jsbmb.2013.02.006. Epub 2013 Feb 19. J Steroid Biochem Mol Biol. 2013. PMID: 23435015 Free PMC article. Review.
Cited by
-
Estrogens in Human Male Gonadotropin Secretion and Testicular Physiology From Infancy to Late Puberty.Front Endocrinol (Lausanne). 2020 Feb 25;11:72. doi: 10.3389/fendo.2020.00072. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32158430 Free PMC article. Review.
-
Alternative pathway androgen biosynthesis and human fetal female virilization.Proc Natl Acad Sci U S A. 2019 Oct 29;116(44):22294-22299. doi: 10.1073/pnas.1906623116. Epub 2019 Oct 14. Proc Natl Acad Sci U S A. 2019. PMID: 31611378 Free PMC article.
-
The effects of selected inhibitors on human fetal adrenal steroidogenesis differs under basal and ACTH-stimulated conditions.BMC Med. 2021 Sep 8;19(1):204. doi: 10.1186/s12916-021-02080-8. BMC Med. 2021. PMID: 34493283 Free PMC article.
-
The Prenatal Hormone Milieu in Autism Spectrum Disorder.Front Psychiatry. 2021 Jul 1;12:655438. doi: 10.3389/fpsyt.2021.655438. eCollection 2021. Front Psychiatry. 2021. PMID: 34276434 Free PMC article. Review.
-
Elevation of corticosterone and 17OH progesterone in extremely preterm infants and clinical implications.Pediatr Res. 2025 Aug 7. doi: 10.1038/s41390-025-04216-5. Online ahead of print. Pediatr Res. 2025. PMID: 40775060
References
-
- Novoselova TV, Jackson D, Campbell DC, Clark AJL, Chan LF. Melanocortin receptor accessory proteins in adrenal gland physiology and beyond. J Endocrinol. 2013;217(1):R1–R11. - PubMed
-
- Ekström L, Rane A. Genetic variation, expression and ontogeny of sulfotransferase SULT2A1 in humans. Pharmacogenomics J. 2015;15(4):293–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

