Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants
- PMID: 30590802
- PMCID: PMC6432864
- DOI: 10.1093/toxsci/kfy309
Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants
Abstract
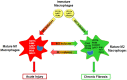
A diverse group of toxicants has been identified that cause injury to the lung including gases (eg, ozone, chlorine), particulates/aerosols (eg, diesel exhaust, fly ash, other combustion products, mustards, nanomaterials, silica, asbestos), chemotherapeutics (eg, bleomycin), and radiation. The pathologic response to these toxicants depends on the dose and duration of exposure and their physical/chemical properties. A common response to pulmonary toxicant exposure is an accumulation of proinflammatory/cytotoxic M1 macrophages at sites of tissue injury, followed by the appearance of anti-inflammatory/wound repair M2 macrophages. It is thought that the outcome of the pathogenic responses to toxicants depends on the balance in the activity of these macrophage subpopulations. Overactivation of either M1 or M2 macrophages leads to injury and disease pathogenesis. Thus, the very same macrophage-derived mediators, released in controlled amounts to destroy injurious materials and pathogens (eg, reactive oxygen species, reactive nitrogen species, proteases, tumor necrosis factor α) and initiate wound repair (eg, transforming growth factor β, connective tissue growth factor, vascular endothelial growth factor), can exacerbate acute lung injury and/or induce chronic disease such as fibrosis, chronic obstructive pulmonary disease, and asthma, when released in excess. This review focuses on the role of macrophage subsets in acute lung injury and chronic fibrosis. Understanding how these pathologies develop following exposure to toxicants, and the contribution of resident and inflammatory macrophages to disease pathogenesis may lead to the development of novel approaches for treating lung diseases.
Keywords: Macrophages; cytokines; fibrosis; inflammatory mediators; lung injury; oxidants.
© The Author(s) 2018. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Characterization of Distinct Macrophage Subpopulations during Nitrogen Mustard-Induced Lung Injury and Fibrosis.Am J Respir Cell Mol Biol. 2016 Mar;54(3):436-46. doi: 10.1165/rcmb.2015-0120OC. Am J Respir Cell Mol Biol. 2016. PMID: 26273949 Free PMC article.
-
Pulmonary toxicants and fibrosis: innate and adaptive immune mechanisms.Toxicol Appl Pharmacol. 2020 Dec 15;409:115272. doi: 10.1016/j.taap.2020.115272. Epub 2020 Oct 5. Toxicol Appl Pharmacol. 2020. PMID: 33031836 Free PMC article. Review.
-
Development of chronic lung injury and pulmonary fibrosis in mice following acute exposure to nitrogen mustard.Inhal Toxicol. 2020 Mar;32(4):141-154. doi: 10.1080/08958378.2020.1757791. Epub 2020 May 3. Inhal Toxicol. 2020. PMID: 32362214
-
Attenuation of Nitrogen Mustard-Induced Pulmonary Injury and Fibrosis by Anti-Tumor Necrosis Factor-α Antibody.Toxicol Sci. 2015 Nov;148(1):71-88. doi: 10.1093/toxsci/kfv161. Epub 2015 Aug 4. Toxicol Sci. 2015. PMID: 26243812 Free PMC article.
-
The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases.Cells. 2021 Apr 14;10(4):897. doi: 10.3390/cells10040897. Cells. 2021. PMID: 33919784 Free PMC article. Review.
Cited by
-
Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome.Inflamm Res. 2020 Sep;69(9):883-895. doi: 10.1007/s00011-020-01378-2. Epub 2020 Jul 10. Inflamm Res. 2020. PMID: 32647933 Free PMC article. Review.
-
Enoxaparin Attenuates Acute Lung Injury and Inflammasome Activation after Traumatic Brain Injury.J Neurotrauma. 2021 Mar;38(5):646-654. doi: 10.1089/neu.2020.7257. Epub 2020 Aug 11. J Neurotrauma. 2021. PMID: 32669032 Free PMC article.
-
[Physical long-term consequences of cancer].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022 Apr;65(4):420-430. doi: 10.1007/s00103-022-03504-3. Epub 2022 Mar 21. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022. PMID: 35312813 Review. German.
-
The Acute Toxicity of Mineral Fibres: A Systematic In Vitro Study Using Different THP-1 Macrophage Phenotypes.Int J Mol Sci. 2022 Mar 4;23(5):2840. doi: 10.3390/ijms23052840. Int J Mol Sci. 2022. PMID: 35269982 Free PMC article.
-
The Biological and Molecular Action of Ozone and Its Derivatives: State-of-the-Art, Enhanced Scenarios, and Quality Insights.Int J Mol Sci. 2023 May 9;24(10):8465. doi: 10.3390/ijms24108465. Int J Mol Sci. 2023. PMID: 37239818 Free PMC article. Review.
References
-
- Al-Harbi N. O., Imam F., Al-Harbi M. M., Ansari M. A., Zoheir K. M. A., Korashy H. M., Sayed-Ahmed M. M., Attia S. M., Shabanah O. A., Ahmad S. F. (2016). Dexamethasone attenuates LPS-induced acute lung injury through inhibition of NF-κB, COX-2, and pro-inflammatory mediators. Immunol. Invest. 45, 349–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

