Fine-Tuning of Sox17 and Canonical Wnt Coordinates the Permeability Properties of the Blood-Brain Barrier
- PMID: 30591003
- PMCID: PMC6407809
- DOI: 10.1161/CIRCRESAHA.118.313316
Fine-Tuning of Sox17 and Canonical Wnt Coordinates the Permeability Properties of the Blood-Brain Barrier
Abstract
Rationale: The microvasculature of the central nervous system includes the blood-brain barrier (BBB), which regulates the permeability to nutrients and restricts the passage of toxic agents and inflammatory cells. Canonical Wnt/β-catenin signaling is responsible for the early phases of brain vascularization and BBB differentiation. However, this signal declines after birth, and other signaling pathways able to maintain barrier integrity at postnatal stage are still unknown.
Objective: Sox17 (SRY [sex-determining region Y]-box 17) constitutes a major downstream target of Wnt/β-catenin in endothelial cells and regulates arterial differentiation. In the present article, we asked whether Sox17 may act downstream of Wnt/β-catenin in inducing BBB differentiation and maintenance.
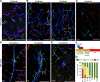
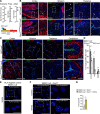
Methods and results: Using reporter mice and nuclear staining of Sox17 and β-catenin, we report that although β-catenin signaling declines after birth, Sox17 activation increases and remains high in the adult. Endothelial-specific inactivation of Sox17 leads to increase of permeability of the brain microcirculation. The severity of this effect depends on the degree of BBB maturation: it is strong in the embryo and progressively declines after birth. In search of Sox17 mechanism of action, RNA sequencing analysis of gene expression of brain endothelial cells has identified members of the Wnt/β-catenin signaling pathway as downstream targets of Sox17. Consistently, we found that Sox17 is a positive inducer of Wnt/β-catenin signaling, and it acts in concert with this pathway to induce and maintain BBB properties. In vivo, inhibition of the β-catenin destruction complex or expression of a degradation-resistant β-catenin mutant, prevent the increase in permeability and retina vascular malformations observed in the absence of Sox17.
Conclusions: Our data highlight a novel role for Sox17 in the induction and maintenance of the BBB, and they underline the strict reciprocal tuning of this transcription factor and Wnt/β-catenin pathway. Modulation of Sox17 activity may be relevant to control BBB permeability in pathological conditions.
Keywords: Wnt/β-catenin; blood-brain barrier; endothelial cells; permeability; stroke.
Figures






References
-
- Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357:eaal2379. doi: 10.1126/science.aal2379. - PubMed
-
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. - PubMed
-
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

