Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer's disease: a network meta-analysis of 41 randomized controlled trials
- PMID: 30591071
- PMCID: PMC6309083
- DOI: 10.1186/s13195-018-0457-9
Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer's disease: a network meta-analysis of 41 randomized controlled trials
Abstract
Background: Cholinesterase inhibitors and memantine have been approved for management of Alzheimer's disease (AD), but there has been no consensus about the choice of various types and doses of drugs at different stages. Hence, we compared and ranked the efficacy and tolerability of these available drugs.
Methods: We searched PubMed, the Cochrane Central Register of Controlled Trials, and Embase for randomized controlled trials (RCTs) published from database inception to July 21, 2017. The primary outcomes were the mean overall changes in cognitive function and responders who had any adverse events. We conducted a random-effects network meta-analysis.
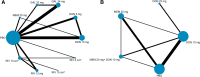
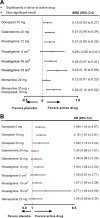
Results: Forty-one RCTs were included in this study. Compared with placebo, galantamine 32 mg daily (standardized mean difference - 0.51, 95% credible interval - 0.67 to - 0.35), galantamine 24 mg daily (- 0.50, - 0.61 to - 0.40), and donepezil 10 mg daily (- 0.40, - 0.51 to - 0.29) were probably the most effective agents on cognition for mild to moderate AD, and memantine 20 mg combined with donepezil 10 mg (0.76, 0.39 to 1.11) was recommended for moderate to severe patients. Memantine showed the best profile of acceptability. Rivastigmine transdermal 15-cm2 patch was the best optional treatment both in function and global changes. None of the medicines was likely to improve neuropsychiatric symptoms through this analysis.
Conclusions: Pharmacological interventions have beneficial effects on cognition, function, and global changes, but not on neuropsychiatric symptoms, through current network meta-analysis. The choice of drugs may mainly depend on the disease severity and clinical symptoms.
Keywords: Alzheimer’s disease; Cholinesterase inhibitors; Memantine; Network meta-analysis.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015—the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015.
-
- NICE . Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. London: National Institute for Health and Care Excellence; 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

