Caloric restriction is associated with preservation of muscle strength in experimental cancer cachexia
- PMID: 30591621
- PMCID: PMC6326673
- DOI: 10.18632/aging.101724
Caloric restriction is associated with preservation of muscle strength in experimental cancer cachexia
Abstract
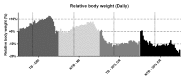
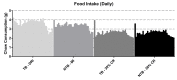
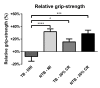
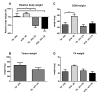
Caloric restriction increases lifespan and healthspan, and limits age-associated muscle wasting. In this study, we investigate the impact of 30% caloric restriction (CR) in a murine cancer cachexia model. Forty CD2F1 mice were allocated as C26 tumor-bearing (TB) + ad libitum food intake (dietary reference intake [DRI]), TB CR, non-TB (NTB) CR, or NTB matched intake (MI). TB groups were inoculated subcutaneously with 0.5x106 C26 cells 14 days after initiating CR. Bodyweight, food intake, and grip-strength were recorded periodically. Gastrocnemius (GCM) and tibialis anterior (TA) muscles were resected and weighed 3 weeks after tumor inoculation. mRNA expression of MuRF1, Atrogin-1, myogenin, and MyoD was determined. At tumor inoculation, the mean body weight of TB CR was 88.6% of initial body weight and remained stable until sacrifice. TB DRI showed wasting before sacrifice. TB groups experienced muscle wasting compared with NTB MI. Grip-strength change was less severe in TB CR. Expression of MuRF1, Atrogin-1, and MyoD was similar between TB DRI and both CR groups. Expression of myogenin was increased in CR groups. In conclusion, caloric restriction limits loss of muscle strength but has no impact on muscle mass despite significant loss of body weight in an experimental cancer-associated cachexia model.
Keywords: cachexia; caloric restriction; cancer; muscle wasting.
Conflict of interest statement
Figures
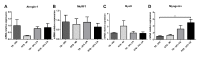
References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, et al.. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011; 12:489–95. 10.1016/S1470-2045(10)70218-7 - DOI - PubMed
-
- Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004; 90:996–1002. 10.1038/sj.bjc.6601620 - DOI - PMC - PubMed
-
- Warren S. The immediate causes of death in cancer. Am J Med Sci. 1932; 184:610–15. 10.1097/00000441-193211000-00002 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

