Structure and Synthesis of Antifungal Disulfide β-Strand Proteins from Filamentous Fungi
- PMID: 30591636
- PMCID: PMC6352176
- DOI: 10.3390/microorganisms7010005
Structure and Synthesis of Antifungal Disulfide β-Strand Proteins from Filamentous Fungi
Abstract
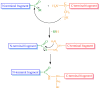
The discovery and understanding of the mode of action of new antimicrobial agents is extremely urgent, since fungal infections cause 1.5 million deaths annually. Antifungal peptides and proteins represent a significant group of compounds that are able to kill pathogenic fungi. Based on phylogenetic analyses the ascomycetous, cysteine-rich antifungal proteins can be divided into three different groups: Penicillium chrysogenum antifungal protein (PAF), Neosartorya fischeri antifungal protein 2 (NFAP2) and "bubble-proteins" (BP) produced, for example, by P. brevicompactum. They all dominantly have β-strand secondary structures that are stabilized by several disulfide bonds. The PAF group (AFP antifungal protein from Aspergillus giganteus, PAF and PAFB from P. chrysogenum, Neosartorya fischeri antifungal protein (NFAP)) is the best characterized with their common β-barrel tertiary structure. These proteins and variants can efficiently be obtained either from fungi production or by recombinant expression. However, chemical synthesis may be a complementary aid for preparing unusual modifications, e.g., the incorporation of non-coded amino acids, fluorophores, or even unnatural disulfide bonds. Synthetic variants up to ca. 6⁻7 kDa can also be put to good use for corroborating structure determination. A short overview of the structural peculiarities of antifungal β-strand disulfide bridged proteins will be given. Here, we describe the structural propensities of some known antifungal proteins from filamentous fungi which can also be prepared with modern synthetic chemistry methods.
Keywords: NFAP2; PAF; antifungal protein; chemical synthesis; disulfide bond; native chemical ligation; solid-phase peptide synthesis; structure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

