Microstructure Optimization of Mg-Alloys by the ECAP Process Including Numerical Simulation, SPD Treatments, Characterization, and Hydrogen Sorption Properties
- PMID: 30591659
- PMCID: PMC6337228
- DOI: 10.3390/molecules24010089
Microstructure Optimization of Mg-Alloys by the ECAP Process Including Numerical Simulation, SPD Treatments, Characterization, and Hydrogen Sorption Properties
Abstract
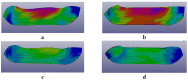
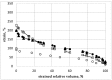
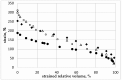
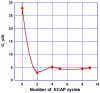
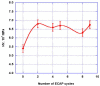
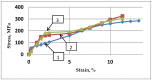
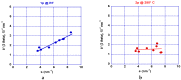
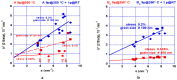
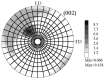
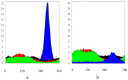
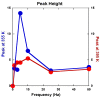
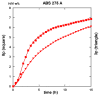
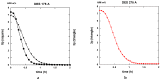
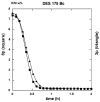
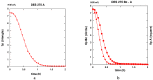
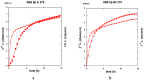
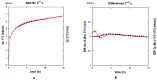
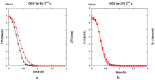
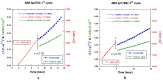
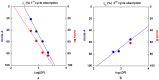
Both numerical simulation and hardness measurements were used to determine the mechanical and microstructural behavior of AZ31 bulk samples when submitted to the Equal Channel Angular Pressing (ECAP) technique. Billets of this representative of Mg-rich alloys were submitted to different numbers of passes for various ECAP modes (anisotropic A, isotropic BC). The strain distribution, the grain size refinement, and the micro-hardness were used as indicators to quantify the effectiveness of the different processing routes. Structural characterizations at different scales were achieved using Scanning Electron Microscopy (SEM), micro-analysis, metallography, Small Angle Neutron Scattering SANS, X-Ray Diffraction (XRD), and texture determination. The grain and crystallite size distribution and orientation as well as defect impacts were determined. Anelastic Spectroscopy (AS) on mechanically deformed samples have shown that the temperature of ECAP differentiate the fragile to ductile regime. MgH₂ consolidated powders were checked for using AS to detect potential hydrogen motions and interaction with host metal atoms. After further optimization, the different mechanically-treated samples were submitted to hydrogenation/dehydrogenation (H/D) cycles, which shows that, for a few passes, the BC mode is better than the A one, as supported by theoretical and experimental microstructure analyses. Accordingly, the hydrogen uptake and (H/D) reactions were correlated with the optimized microstructure peculiarities and interpreted in terms of Johnson-Avrami- Mehl-Kolmogorov (JAMK) and Jander models, successively.
Keywords: ECAP process; anelastic spectroscopy; grain and crystallite size; hydrogen uptake and kinetics sorption; magnesium alloys; numerical simulation; severe plastic deformation; texture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
-
- Zehetbauer M.J., Valiev R.Z. Nanomaterials by Severe Plastic Deformation. Wiley-VCH GmbH; Weinheim, Germany: 2004. p. 850. - DOI
-
- Kim H.S., Estrin Y. Microstructural modelling of equal channel angular pressing for producing ultrafine grained materials. J. Mater. Process. Technol. 2005;410–411:285–289. doi: 10.1016/j.msea.2005.08.047. - DOI
-
- Figueiredo R., Langdon T. Grain refinement and mechanical behavior of a magnesium alloy processed by ECAP. J. Mater. Sci. 2010;45:4827–4836. doi: 10.1007/s10853-010-4589-y. - DOI
-
- Young J.P., Askari H., Hovanski Y., Heiden M.J., Field D.P. Microstructural stability after severe plastic deformation of AZ31 Magnesium. Mater. Charact. 2015;101:9–19. doi: 10.1016/j.matchar.2014.12.026. - DOI
-
- Yurchenkoa N.Y., Stepanova N.D., Salishcheva G.A., Rokhlinb L.L., Rokhlinb S.V. Effect of multiaxial forging on microstructure and mechanical properties of Mg-0.8Ca alloy. IOP Conf. Ser. Mater. Sci. Eng. 2014;63:012075. doi: 10.1088/1757-899X/63/1/012075. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

