Effects of Novel Calpain Inhibitors in Transgenic Animal Model of Parkinson's disease/dementia with Lewy bodies
- PMID: 30591714
- PMCID: PMC6308237
- DOI: 10.1038/s41598-018-35729-1
Effects of Novel Calpain Inhibitors in Transgenic Animal Model of Parkinson's disease/dementia with Lewy bodies
Abstract
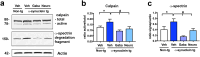
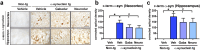
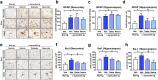
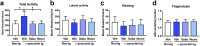
Parkinson's disease (PD) and dementia with Lewy bodies (DLB) are neurodegenerative disorders of the aging population characterized by the accumulation of α-synuclein (α-syn). The mechanisms triggering α-syn toxicity are not completely understood, however, c-terminus truncation of α-syn by proteases such as calpain may have a role. Therefore, inhibition of calpain may be of value. The main objective of this study was to evaluate the effects of systemically administered novel low molecular weight calpain inhibitors on α-syn pathology in a transgenic mouse model. For this purpose, non-tg and α-syn tg mice received the calpain inhibitors - Gabadur, Neurodur or a vehicle, twice a day for 30 days. Immunocytochemical analysis showed a 60% reduction in α-syn deposition using Gabadur and a 40% reduction using Neurodur with a concomitant reduction in c-terminus α-syn and improvements in neurodegeneration. Western blot analysis showed a 77% decrease in α-spectrin breakdown products (SBDPs) SBDPs with Gabadur and 63% reduction using Neurodur. There was a 65% reduction in the active calpain form with Gabadur and a 45% reduction with Neurodur. Moreover, treatment with calpain inhibitors improved activity performance of the α-syn tg mice. Taken together, this study suggests that calpain inhibition might be considered in the treatment of synucleinopathies.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Alafuzoff, I. & Hartikainen, P. Alpha-synucleinopathies. Handb Clin Neurol145, 339–353, 10.1016/B978-0-12-802395-2.00024-9 (2017). - PubMed
-
- Jellinger, K. A. Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm (Vienna), 10.1007/s00702-017-1821-9 (2017). - PubMed
-
- Ono, K. The Oligomer Hypothesis in alpha-Synucleinopathy. Neurochem Res42, 3362–3371, 10.1007/s11064-017-2382-x (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

