Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04)
- PMID: 30591866
- PMCID: PMC6303395
- DOI: 10.1016/j.jbo.2018.09.008
Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04)
Abstract
Adjuvant bisphosphonates improve disease outcomes in postmenopausal early breast cancer (EBC) but the long-term effects are poorly described. The AZURE trial (ISRCTN79831382) was designed to determine whether adjuvant zoledronic acid (ZOL) improves disease outcomes in EBC. Previous analyses showed no effect on overall outcomes but identified benefits in postmenopausal women. Here we present the long-term risks and benefits of adjuvant ZOL with 10-years follow-up.
Patients and methods: 3360 patients with stage II/III breast cancer were included in an academic, international, phase III, randomized, open label trial. Patients were followed up on a regular schedule until 10 years. Patients were randomized on a 1:1 basis to standard adjuvant systemic therapy +/- intravenous ZOL 4 mg every 3-4 weeks x6, and then at reduced frequency to complete 5 years treatment. The primary outcome was disease free survival (DFS). Secondary outcomes included invasive DFS (IDFS), overall survival (OS), sites of recurrence, skeletal morbidity and treatment outcomes according to primary tumor amplification of the transcription factor, MAF. Pre-planned subgroup analyses focused on interactions between menopausal status and treatment effects.
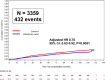
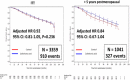
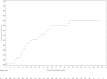
Results: With a median follow up of 117 months [IQR 70.4-120.4), DFS and IDFS were similar in both arms (HRDFS = 0.94, 95%CI = 0.84-1.06, p = 0.340; HRIDFS = 0.91, 95%CI = 0.82-1.02, p = 0.116). However, outcomes remain improved with ZOL in postmenopausal women (HRDFS = 0.82, 95%CI = 0.67-1.00; HRIDFS = 0.78, 95%CI = 0.64-0.94). In the 79% of tested women with a MAF FISH negative tumor, ZOL improved IDFS (HRIDFS = 0.75, 95%CI = 0.58-0.97) and OS HROS = 0.69, 95%CI = 0.50-0.94), irrespective of menopause. ZOL did not improve disease outcomes in MAF FISH + tumors. Bone metastases as a first DFS recurrence (BDFS) were reduced with ZOL (HRB-DFS = 0.76, 95%CI = 0.63-0.92, p = 0.005). ZOL reduced skeletal morbidity with fewer fractures and skeletal events after disease recurrence. 30 cases of osteonecrosis of the jaw in the ZOL arm (1.8%) have occurred.
Conclusions: Disease benefits with adjuvant ZOL in postmenopausal early breast cancer persist at 10 years of follow-up. The biomarker MAF identified a patient subgroup that derived benefit from ZOL irrespective of menopausal status.
Figures





References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Allen C., Barber R.M. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. - PMC - PubMed
-
- Ursini-Siegel J., Siegel P.M. The influence of the pre-metastatic niche on breast cancer metastasis. Cancer Lett. 2016;380(1):281–288. - PubMed
-
- Coleman RE., Marshall H., Cameron D., et al on behalf of the AZURE investigators Breast cancer adjuvant therapy with zoledronic acid. New Engl. J. Med. 2011;365:1396–1405. - PubMed
-
- Coleman R.E., Cameron D., Dodwell D. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomized open-label phase 3 trial. Lancet Oncol. 2014;15:997–1006. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

