Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye
- PMID: 30591984
- PMCID: PMC6308217
- DOI: 10.1007/s11095-018-2554-7
Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye
Abstract
A resurgence of interest and investment in the field of gene therapy, driven in large part by advances in viral vector technology, has recently culminated in United States Food and Drug Administration approval of the first gene therapy product targeting a disease caused by mutations in a single gene. This product, LUXTURNA™ (voretigene neparvovec-rzyl; Spark Therapeutics, Inc., Philadelphia, PA), delivers a normal copy of the RPE65 gene to retinal cells for the treatment of biallelic RPE65 mutation-associated retinal dystrophy, a blinding disease. Many additional gene therapy programs targeting both inherited retinal diseases and other ocular diseases are in development, owing to an improved understanding of the genetic basis of ocular disease and the unique properties of the ocular compartment that make it amenable to local gene therapy. Here we review the growing body of literature that describes both the design and development of ocular gene therapy products, with a particular emphasis on target and vector selection, and chemistry, manufacturing, and controls.
Keywords: adeno-associated virus (AAV) vector; formulation; gene therapy; ocular diseases; product development.
Figures
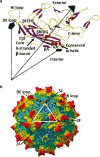



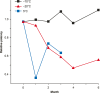


References
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. - DOI - PubMed
-
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. - DOI - PMC - PubMed
-
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

