Current concepts in cochlear ribbon synapse formation
- PMID: 30592086
- PMCID: PMC6573016
- DOI: 10.1002/syn.22087
Current concepts in cochlear ribbon synapse formation
Abstract
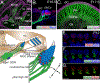
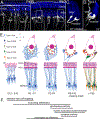
In mammals, hair cells and spiral ganglion neurons (SGNs) in the cochlea together are sophisticated "sensorineural" structures that transduce auditory information from the outside world into the brain. Hair cells and SGNs are joined by glutamatergic ribbon-type synapses composed of a molecular machinery rivaling in complexity the mechanoelectric transduction components found at the apical side of the hair cell. The cochlear hair cell ribbon synapse has received much attention lately because of recent and important findings related to its damage (sometimes termed "synaptopathy") as a result of noise overexposure. During development, ribbon synapses between type I SGNs and inner hair cells form in the time window between birth and hearing onset and is a process coordinated with type I SGN myelination, spontaneous activity, synaptic pruning, and innervation by efferents. In this review, we highlight new findings regarding the diversity of type I SGNs and inner hair cell synapses, and the molecular mechanisms of selective hair cell targeting. Also discussed are cell adhesion molecules and protein constituents of the ribbon synapse, and how these factors participate in ribbon synapse formation. We also note interesting new insights into the morphological development of type II SGNs, and the potential for cochlear macrophages as important players in protecting SGNs. We also address recent studies demonstrating that the structural and physiological profiles of the type I SGNs do not reach full maturity until weeks after hearing onset, suggesting a protracted development that is likely modulated by activity.
Keywords: cochlea; hair cell; ribbon synapse; spiral ganglion neuron; synaptopathy.
© 2018 Wiley Periodicals, Inc.
Figures
References
-
- Adamson CL, Reid M a, & Davis RL (2002). Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience doi:22/4/1385 [pii] - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
