Two central pattern generators from the crab, Cancer borealis, respond robustly and differentially to extreme extracellular pH
- PMID: 30592258
- PMCID: PMC6328273
- DOI: 10.7554/eLife.41877
Two central pattern generators from the crab, Cancer borealis, respond robustly and differentially to extreme extracellular pH
Abstract
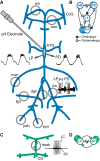
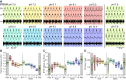
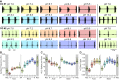
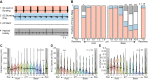
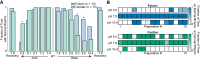
The activity of neuronal circuits depends on the properties of the constituent neurons and their underlying synaptic and intrinsic currents. We describe the effects of extreme changes in extracellular pH - from pH 5.5 to 10.4 - on two central pattern generating networks, the stomatogastric and cardiac ganglia of the crab, Cancer borealis. Given that the physiological properties of ion channels are known to be sensitive to pH within the range tested, it is surprising that these rhythms generally remained robust from pH 6.1 to pH 8.8. The pH sensitivity of these rhythms was highly variable between animals and, unexpectedly, between ganglia. Animal-to-animal variability was likely a consequence of similar network performance arising from variable sets of underlying conductances. Together, these results illustrate the potential difficulty in generalizing the effects of environmental perturbation across circuits, even within the same animal.
Keywords: Cancer borealis; cardiac ganglion; crustacean; neuroscience; ocean acidification; pyloric rhythm; stomatogastric ganglion.
© 2018, Haley et al.
Conflict of interest statement
JH, DH No competing interests declared, EM Deputy Editor, eLife
Figures







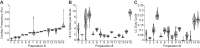

Similar articles
-
Graded Transmission without Action Potentials Sustains Rhythmic Activity in Some But Not All Modulators That Activate the Same Current.J Neurosci. 2018 Oct 17;38(42):8976-8988. doi: 10.1523/JNEUROSCI.2632-17.2018. Epub 2018 Sep 5. J Neurosci. 2018. PMID: 30185461 Free PMC article.
-
Rapid adaptation to elevated extracellular potassium in the pyloric circuit of the crab, Cancer borealis.J Neurophysiol. 2020 May 1;123(5):2075-2089. doi: 10.1152/jn.00135.2020. Epub 2020 Apr 22. J Neurophysiol. 2020. PMID: 32319837 Free PMC article.
-
How can motor systems retain performance over a wide temperature range? Lessons from the crustacean stomatogastric nervous system.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Sep;201(9):851-6. doi: 10.1007/s00359-014-0975-2. Epub 2015 Jan 1. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 25552317 Free PMC article. Review.
-
Pyloric motor pattern modification by a newly identified projection neuron in the crab stomatogastric nervous system.J Neurophysiol. 1996 Jan;75(1):97-108. doi: 10.1152/jn.1996.75.1.97. J Neurophysiol. 1996. PMID: 8822544
-
Modulation of stomatogastric rhythms.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009 Nov;195(11):989-1009. doi: 10.1007/s00359-009-0483-y. Epub 2009 Oct 11. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009. PMID: 19823843 Review.
Cited by
-
Energy-efficient network activity from disparate circuit parameters.Proc Natl Acad Sci U S A. 2022 Nov;119(44):e2207632119. doi: 10.1073/pnas.2207632119. Epub 2022 Oct 24. Proc Natl Acad Sci U S A. 2022. PMID: 36279461 Free PMC article.
-
Hibernation reduces GABA signaling in the brainstem to enhance motor activity of breathing at cool temperatures.BMC Biol. 2024 Nov 4;22(1):251. doi: 10.1186/s12915-024-02050-5. BMC Biol. 2024. PMID: 39497096 Free PMC article.
-
Dimensionality reduction of neuronal degeneracy reveals two interfering physiological mechanisms.PNAS Nexus. 2024 Sep 19;3(10):pgae415. doi: 10.1093/pnasnexus/pgae415. eCollection 2024 Oct. PNAS Nexus. 2024. PMID: 39359396 Free PMC article.
-
The differential contribution of pacemaker neurons to synaptic transmission in the pyloric network of the Jonah crab, Cancer borealis.J Neurophysiol. 2019 Oct 1;122(4):1623-1633. doi: 10.1152/jn.00038.2019. Epub 2019 Aug 14. J Neurophysiol. 2019. PMID: 31411938 Free PMC article.
-
Activity-dependent compensation of cell size is vulnerable to targeted deletion of ion channels.Sci Rep. 2020 Sep 29;10(1):15989. doi: 10.1038/s41598-020-72977-6. Sci Rep. 2020. PMID: 32994529 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

